Calcium and Magnesium Ions Are Membrane-Active against Stationary-Phase Staphylococcus aureus with High Specificity
- PMID: 26865182
- PMCID: PMC4749956
- DOI: 10.1038/srep20628
Calcium and Magnesium Ions Are Membrane-Active against Stationary-Phase Staphylococcus aureus with High Specificity
Abstract
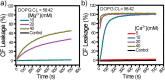
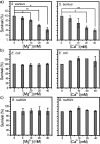
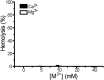
Staphylococcus aureus (S. aureus) is notorious for its ability to acquire antibiotic-resistance, and antibiotic-resistant S. aureus has become a wide-spread cause of high mortality rate. Novel antimicrobials capable of eradicating S. aureus cells including antibiotic-resistant ones are thus highly desired. Membrane-active bactericides and species-specific antimicrobials are two promising sources of novel anti-infective agents for fighting against bacterial antibiotic-resistance. We herein show that Ca(2+) and Mg(2+), two alkaline-earth-metal ions physiologically essential for diverse living organisms, both disrupt model S. aureus membranes and kill stationary-phase S. aureus cells, indicative of membrane-activity. In contrast to S. aureus, Escherichia coli and Bacillus subtilis exhibit unaffected survival after similar treatment with these two cations, indicative of species-specific activity against S. aureus. Moreover, neither Ca(2+) nor Mg(2+) lyses mouse red blood cells, indicative of hemo-compatibility. This works suggests that Ca(2+) and Mg(2+) may have implications in targeted eradication of S. aureus pathogen including the antibiotic-resistant ones.
Figures

Similar articles
-
Bacteria May Cope Differently from Similar Membrane Damage Caused by the Australian Tree Frog Antimicrobial Peptide Maculatin 1.1.J Biol Chem. 2015 Aug 7;290(32):19853-62. doi: 10.1074/jbc.M115.643262. Epub 2015 Jun 22. J Biol Chem. 2015. PMID: 26100634 Free PMC article.
-
Salt-resistant short antimicrobial peptides.Biopolymers. 2016 May;106(3):345-56. doi: 10.1002/bip.22819. Biopolymers. 2016. PMID: 26849911
-
Arginine-lysine positional swap of the LL-37 peptides reveals evolutional advantages of the native sequence and leads to bacterial probes.Biochim Biophys Acta Biomembr. 2017 Aug;1859(8):1350-1361. doi: 10.1016/j.bbamem.2017.04.018. Epub 2017 Apr 24. Biochim Biophys Acta Biomembr. 2017. PMID: 28450045 Free PMC article.
-
In vitro antibacterial properties of magnesium metal against Escherichia coli, Pseudomonas aeruginosa and Staphylococcus aureus.Acta Biomater. 2010 May;6(5):1869-77. doi: 10.1016/j.actbio.2009.10.007. Epub 2009 Oct 7. Acta Biomater. 2010. PMID: 19818422
-
Targeting cell membrane adaptation as a novel antimicrobial strategy.Curr Opin Microbiol. 2016 Oct;33:91-96. doi: 10.1016/j.mib.2016.07.002. Epub 2016 Jul 25. Curr Opin Microbiol. 2016. PMID: 27458841 Free PMC article. Review.
Cited by
-
Multifunctional Hemostatic PVA/Chitosan Sponges Loaded with Hydroxyapatite and Ciprofloxacin.ACS Omega. 2022 Apr 11;7(15):13210-13220. doi: 10.1021/acsomega.2c00654. eCollection 2022 Apr 19. ACS Omega. 2022. PMID: 35474822 Free PMC article.
-
Sintered and 3D-Printed Bulks of MgB2-Based Materials with Antimicrobial Properties.Molecules. 2021 Oct 6;26(19):6045. doi: 10.3390/molecules26196045. Molecules. 2021. PMID: 34641589 Free PMC article.
-
Nanoarchitectonics of Bactericidal Coatings Based on CaCO3-Nanosilver Hybrids.ACS Appl Bio Mater. 2024 May 20;7(5):2872-2886. doi: 10.1021/acsabm.3c01228. Epub 2024 May 9. ACS Appl Bio Mater. 2024. PMID: 38721671 Free PMC article.
-
Calcium phosphate nanoparticles as intrinsic inorganic antimicrobials: In search of the key particle property.Biointerphases. 2019 May 20;14(3):031001. doi: 10.1116/1.5090396. Biointerphases. 2019. PMID: 31109162 Free PMC article.
-
Harzianic Acid Activity against Staphylococcus aureus and Its Role in Calcium Regulation.Toxins (Basel). 2023 Mar 24;15(4):237. doi: 10.3390/toxins15040237. Toxins (Basel). 2023. PMID: 37104175 Free PMC article.
References
-
- Moore C. E., Segal S., Berendt A. R., Hill A. V. S. & Day N. P. J. Lack of association between toll-like receptor 2 polymorphisms and susceptibility to severe disease caused by Staphylococcus aureus. Clin. Diagn. Lab. Immunol. 11, 1194–1197, doi: 10.1128/Cdli.11.6.1194-1197.2004 (2004). - DOI - PMC - PubMed
-
- Wertheim H. F. L. & Verbrugh H. A. Global prevalence of meticillin-resistant Staphylococcus aureus. Lancet 368, 1866 (2006). - PubMed
-
- Antibiotic Resistance Threats in the United States, 2013. Report by the U.S. Centers for Dieseas Control and Prevention.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

