Patterns and Correlates of Baseline Thiazide-Type Diuretic Prescription in the Systolic Blood Pressure Intervention Trial
- PMID: 26865200
- PMCID: PMC4755350
- DOI: 10.1161/HYPERTENSIONAHA.115.06851
Patterns and Correlates of Baseline Thiazide-Type Diuretic Prescription in the Systolic Blood Pressure Intervention Trial
Abstract
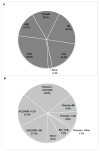
Thiazides and thiazide-type diuretics are recommended as first-line agents for the treatment of hypertension, but contemporary information on their use in clinical practice is lacking. We examined patterns and correlates of thiazide prescription in a cross-sectional analysis of baseline data from participants enrolled in the Systolic Blood Pressure Intervention Trial (SPRINT). We examined baseline prescription of thiazides in 7582 participants receiving at least 1 antihypertensive medication by subgroup, and used log-binomial regression to calculate adjusted prevalence ratios for thiazide prescription (versus no thiazide). Forty-three percent of all participants were prescribed a thiazide at baseline, but among participants prescribed a single agent, the proportion was only 16%. The prevalence of thiazide prescription differed significantly by demographic factors, with younger participants, women, and blacks all having higher adjusted prevalence of thiazide prescription than other corresponding subgroups. Participants in the lowest category of kidney function (estimated glomerular filtration rate <30 mL/min per 1.73 m2) were half as likely to be prescribed a thiazide as participants with preserved kidney function. In conclusion, among persons with hypertension and heightened cardiovascular risk, we found that thiazide prescription varied significantly by demographics and kidney disease status, despite limited evidence about relative differences in effectiveness.
Trial registration: ClinicalTrials.gov NCT01206062.
Keywords: antihypertensive agents; blood pressure; hypertension; risk factors; thiazides.
© 2016 American Heart Association, Inc.
Figures

References
-
- Daskalopoulou SS, Khan NA, Quinn RR, et al. The 2012 Canadian hypertension education program recommendations for the management of hypertension: blood pressure measurement, diagnosis, assessment of risk, and therapy. Can J Cardiol. 2012;28:270–287. - PubMed
-
- Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ. The Seventh Report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. - PubMed
-
- Aronow WS, Fleg JL, Pepine CJ, et al. ACCF/AHA 2011 expert consensus document on hypertension in the elderly: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus documents developed in collaboration with the American Academy of Neurology, American Geriatrics Society, American Society for Preventive Cardiology, American Society of Hypertension, American Society of Nephrology, Association of Black Cardiologists, and European Society of Hypertension. J Am Coll Cardiol. 2011;57:2037–2114. - PubMed
-
- ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) JAMA. 2002;288:2981–2997. - PubMed
-
- ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Diuretic versus alpha-blocker as first-step antihypertensive therapy: final results from the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) Hypertension. 2003;42:239–246. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- UL1TR000433/TR/NCATS NIH HHS/United States
- UL1RR025752/RR/NCRR NIH HHS/United States
- UL1 TR000093/TR/NCATS NIH HHS/United States
- UL1TR000005/TR/NCATS NIH HHS/United States
- K23 DK095914/DK/NIDDK NIH HHS/United States
- P30GM103337/GM/NIGMS NIH HHS/United States
- UL1 TR000050/TR/NCATS NIH HHS/United States
- UL1 RR025755/RR/NCRR NIH HHS/United States
- UL1 TR001085/TR/NCATS NIH HHS/United States
- UL1TR000439/TR/NCATS NIH HHS/United States
- UL1TR000075/TR/NCATS NIH HHS/United States
- UL1 RR024134/RR/NCRR NIH HHS/United States
- UL1 TR000433/TR/NCATS NIH HHS/United States
- UL1 TR001070/TR/NCATS NIH HHS/United States
- UL1TR000050/TR/NCATS NIH HHS/United States
- 9U54TR000017-06/TR/NCATS NIH HHS/United States
- UL1 TR000445/TR/NCATS NIH HHS/United States
- HHSN268200900048C/HL/NHLBI NIH HHS/United States
- UL1 TR000005/TR/NCATS NIH HHS/United States
- HHSN268200900040C/HL/NHLBI NIH HHS/United States
- UL1TR000093/TR/NCATS NIH HHS/United States
- HHSN268200900046C/HL/NHLBI NIH HHS/United States
- UL1 TR000064/TR/NCATS NIH HHS/United States
- UL1TR000073/TR/NCATS NIH HHS/United States
- UL1RR025755/RR/NCRR NIH HHS/United States
- UL1 TR000075/TR/NCATS NIH HHS/United States
- UL1RR025771/RR/NCRR NIH HHS/United States
- P30 GM103337/GM/NIGMS NIH HHS/United States
- UL1 TR001064/TR/NCATS NIH HHS/United States
- UL1 RR025752/RR/NCRR NIH HHS/United States
- UL1TR000105-05/TR/NCATS NIH HHS/United States
- UL1 RR025771/RR/NCRR NIH HHS/United States
- HHSN268200900049C/HL/NHLBI NIH HHS/United States
- HHSN268200900047C/HL/NHLBI NIH HHS/United States
- UL1 TR001427/TR/NCATS NIH HHS/United States
- UL1 TR000003/TR/NCATS NIH HHS/United States
- UL1TR000003/TR/NCATS NIH HHS/United States
- UL1 TR000439/TR/NCATS NIH HHS/United States
- UL1 TR000073/TR/NCATS NIH HHS/United States
- UL1 TR000002/TR/NCATS NIH HHS/United States
- UL1 TR000105/TR/NCATS NIH HHS/United States
- UL1TR001064/TR/NCATS NIH HHS/United States
- UL1RR024134/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

