The effect of disease on the evolution of females and the genetic basis of sex in populations with cytoplasmic male sterility
- PMID: 26865308
- PMCID: PMC4760181
- DOI: 10.1098/rspb.2015.3035
The effect of disease on the evolution of females and the genetic basis of sex in populations with cytoplasmic male sterility
Abstract
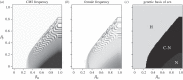
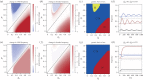
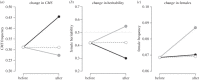
The evolution of separate males and females is an important evolutionary transition that has occurred multiple times in flowering plants. While empirical studies have stressed the potential importance of natural enemies and organismal interactions in the evolution of separate sexes, there has been no treatment of natural enemies in the theoretical literature. We investigated the effects of disease on the evolution of females in gynodioecious populations composed of females and hermaphrodites, where sex is determined by the interaction of cytoplasmic male sterility (CMS) and nuclear restorer genes. When females are significantly more resistant than hermaphrodites, disease drives an increase in the frequency of females and sex determination becomes nuclear, creating the pre-conditions for the evolution of separate males and females. However, when females are only moderately more resistant, disease drives changes in the frequency of CMS and restorer alleles, but has little effect on the frequency of females. We discuss our results in the context of the evolution of mating systems and cyto-nuclear epistasis.
Keywords: cyto-nuclear epistasis; cytoplasmic male sterility; dioecy; disease; gynodioecy; sex-specific resistance.
© 2016 The Author(s).
Figures
Similar articles
-
Gynodioecy to dioecy: are we there yet?Ann Bot. 2012 Feb;109(3):531-43. doi: 10.1093/aob/mcr170. Epub 2011 Aug 1. Ann Bot. 2012. PMID: 21807691 Free PMC article. Review.
-
Evolution of dioecy: can nuclear-cytoplasmic interactions select for maleness?Heredity (Edinb). 1994 Oct;73 ( Pt 4):346-54. doi: 10.1038/hdy.1994.181. Heredity (Edinb). 1994. PMID: 7989215
-
Modeling gynodioecy: novel scenarios for maintaining polymorphism.Am Nat. 2003 May;161(5):762-76. doi: 10.1086/374803. Epub 2003 May 2. Am Nat. 2003. PMID: 12858283
-
NUCLEO-CYTOPLASMIC MALE STERILITY AND ALTERNATIVE ROUTES TO DIOECY.Evolution. 1994 Dec;48(6):1933-1945. doi: 10.1111/j.1558-5646.1994.tb02224.x. Evolution. 1994. PMID: 28565167
-
A conflict between two sexes, females and hermaphrodites.Experientia Suppl. 1987;55:245-61. doi: 10.1007/978-3-0348-6273-8_11. Experientia Suppl. 1987. PMID: 2961599 Review.
Cited by
-
Vector preference and heterogeneity in host sex ratio can affect pathogen spread in natural plant populations.Ecology. 2021 Mar;102(3):e03246. doi: 10.1002/ecy.3246. Epub 2021 Jan 18. Ecology. 2021. PMID: 33190245 Free PMC article.
-
The role of infectious disease in the evolution of females: Evidence from anther-smut disease on a gynodioecious alpine carnation.Evolution. 2019 Mar;73(3):497-510. doi: 10.1111/evo.13640. Epub 2018 Nov 28. Evolution. 2019. PMID: 30411338 Free PMC article.
-
Current understanding of male sterility systems in vegetable Brassicas and their exploitation in hybrid breeding.Plant Reprod. 2019 Sep;32(3):231-256. doi: 10.1007/s00497-019-00371-y. Epub 2019 May 3. Plant Reprod. 2019. PMID: 31053901 Review.
References
-
- Hamilton WD. 1980. Sex versus non-sex versus parasite. Oikos 35, 282–290. (10.2307/3544435) - DOI
-
- Lively CM. 1996. Host-parasite coevolution and sex. Bioscience 46, 107–114. (10.2307/1312813) - DOI
-
- Lively CM, Craddock C, Vrijenhoek RC. 1990. Red queen hypothesis supported by parasitism in sexual and clonal fish. Nature 344, 864–866. (10.1038/344864a0) - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

