Triazine-Based Sequence-Defined Polymers with Side-Chain Diversity and Backbone-Backbone Interaction Motifs
- PMID: 26865312
- PMCID: PMC4804744
- DOI: 10.1002/anie.201509864
Triazine-Based Sequence-Defined Polymers with Side-Chain Diversity and Backbone-Backbone Interaction Motifs
Abstract
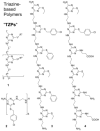
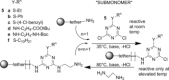
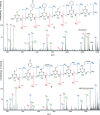
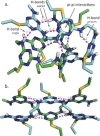
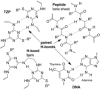
Sequence control in polymers, well-known in nature, encodes structure and functionality. Here we introduce a new architecture, based on the nucleophilic aromatic substitution chemistry of cyanuric chloride, that creates a new class of sequence-defined polymers dubbed TZPs. Proof of concept is demonstrated with two synthesized hexamers, having neutral and ionizable side chains. Molecular dynamics simulations show backbone-backbone interactions, including H-bonding motifs and pi-pi interactions. This architecture is arguably biomimetic while differing from sequence-defined polymers having peptide bonds. The synthetic methodology supports the structural diversity of side chains known in peptides, as well as backbone-backbone hydrogen-bonding motifs, and will thus enable new macromolecules and materials with useful functions.
Keywords: biomimetics; macromolecules; sequence-defined polymers; simulations; solid-phase synthesis.
© 2016 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures



Similar articles
-
Foldamer Architectures of Triazine-Based Sequence-Defined Polymers Investigated with Molecular Dynamics Simulations and Enhanced Sampling Methods.J Phys Chem B. 2019 Nov 7;123(44):9364-9377. doi: 10.1021/acs.jpcb.9b06067. Epub 2019 Oct 24. J Phys Chem B. 2019. PMID: 31603686
-
Investigating the role of non-covalent interactions in conformation and assembly of triazine-based sequence-defined polymers.J Chem Phys. 2018 Aug 21;149(7):072330. doi: 10.1063/1.5024552. J Chem Phys. 2018. PMID: 30134719
-
Exploration of Tertiary Structure in Sequence-Defined Polymers Using Molecular Dynamics Simulations.Biomacromolecules. 2024 Oct 14;25(10):6439-6450. doi: 10.1021/acs.biomac.4c00527. Epub 2024 Sep 18. Biomacromolecules. 2024. PMID: 39292171
-
Routes to Hydrogen Bonding Chain-End Functionalized Polymers.Macromol Rapid Commun. 2012 Dec 21;33(24):2062-91. doi: 10.1002/marc.201200508. Epub 2012 Nov 7. Macromol Rapid Commun. 2012. PMID: 23136120 Review.
-
Recent Advances of Triazine-Based Materials for Adsorbent Based Extraction Techniques.Top Curr Chem (Cham). 2021 May 4;379(4):24. doi: 10.1007/s41061-021-00336-8. Top Curr Chem (Cham). 2021. PMID: 33945059 Review.
Cited by
-
Dynamic Bonds: Adaptable Timescales for Responsive Materials.Angew Chem Int Ed Engl. 2022 Dec 12;61(50):e202206938. doi: 10.1002/anie.202206938. Epub 2022 Nov 2. Angew Chem Int Ed Engl. 2022. PMID: 36167937 Free PMC article. Review.
-
Discrete Oligocarbamates Exhibit Sequence-Dependent Fluorescence Emission and Quenching.ACS Polym Au. 2023 Mar 5;3(3):276-283. doi: 10.1021/acspolymersau.2c00070. eCollection 2023 Jun 14. ACS Polym Au. 2023. PMID: 37334195 Free PMC article.
-
Coding and decoding libraries of sequence-defined functional copolymers synthesized via photoligation.Nat Commun. 2016 Nov 30;7:13672. doi: 10.1038/ncomms13672. Nat Commun. 2016. PMID: 27901024 Free PMC article.
-
Sequence-Controlled Polymers Through Entropy-Driven Ring-Opening Metathesis Polymerization: Theory, Molecular Weight Control, and Monomer Design.J Am Chem Soc. 2019 Apr 10;141(14):5741-5752. doi: 10.1021/jacs.8b13120. Epub 2019 Mar 27. J Am Chem Soc. 2019. PMID: 30714723 Free PMC article.
-
Efficient automated solid-phase synthesis of recognition-encoded melamine oligomers.Chem Sci. 2024 Mar 27;15(16):5957-5963. doi: 10.1039/d4sc00973h. eCollection 2024 Apr 24. Chem Sci. 2024. PMID: 38665524 Free PMC article.
References
-
- None
-
- Sequence-Controlled Polymers: Synthesis Self-Assembly, and Properties, Vol. 1170, American Chemical Society, Washington, D.C., 2014;
-
- Lutz J.-F., Ouchi M., Liu D. R., Sawamoto M., Science 2013, 341, 1238149; - PubMed
-
- Stayshich R. M., Weiss R. M., Li J., Meyer T. Y., Macromol. Rapid Commun. 2011, 32, 220–225; - PubMed
-
- Norris B. N., Zhang S., Campbell C. M., Auletta J. T., Calvo-Marzal P., Hutchison G. R., Meyer T. Y., Macromolecules 2013, 46, 1384–1392;
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

