The use of a gas chromatography-sensor system combined with advanced statistical methods, towards the diagnosis of urological malignancies
- PMID: 26865331
- PMCID: PMC4876927
- DOI: 10.1088/1752-7155/10/1/017106
The use of a gas chromatography-sensor system combined with advanced statistical methods, towards the diagnosis of urological malignancies
Abstract
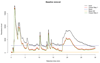
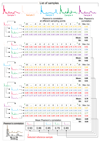
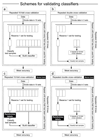
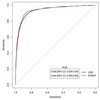
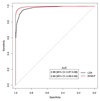
Prostate cancer is one of the most common cancers. Serum prostate-specific antigen (PSA) is used to aid the selection of men undergoing biopsies. Its use remains controversial. We propose a GC-sensor algorithm system for classifying urine samples from patients with urological symptoms. This pilot study includes 155 men presenting to urology clinics, 58 were diagnosed with prostate cancer, 24 with bladder cancer and 73 with haematuria and or poor stream, without cancer. Principal component analysis (PCA) was applied to assess the discrimination achieved, while linear discriminant analysis (LDA) and support vector machine (SVM) were used as statistical models for sample classification. Leave-one-out cross-validation (LOOCV), repeated 10-fold cross-validation (10FoldCV), repeated double cross-validation (DoubleCV) and Monte Carlo permutations were applied to assess performance. Significant separation was found between prostate cancer and control samples, bladder cancer and controls and between bladder and prostate cancer samples. For prostate cancer diagnosis, the GC/SVM system classified samples with 95% sensitivity and 96% specificity after LOOCV. For bladder cancer diagnosis, the SVM reported 96% sensitivity and 100% specificity after LOOCV, while the DoubleCV reported 87% sensitivity and 99% specificity, with SVM showing 78% and 98% sensitivity between prostate and bladder cancer samples. Evaluation of the results of the Monte Carlo permutation of class labels obtained chance-like accuracy values around 50% suggesting the observed results for bladder cancer and prostate cancer detection are not due to over fitting. The results of the pilot study presented here indicate that the GC system is able to successfully identify patterns that allow classification of urine samples from patients with urological cancers. An accurate diagnosis based on urine samples would reduce the number of negative prostate biopsies performed, and the frequency of surveillance cystoscopy for bladder cancer patients. Larger cohort studies are planned to investigate the potential of this system. Future work may lead to non-invasive breath analyses for diagnosing urological conditions.
Figures

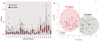

References
-
- [Accessed June 6 2015];Cancer Research UK Web site. http://www.cancerresearchuk.org/cancer-info/cancerstats/types/bladder/in...
-
- Garg V, Gu NY, Borrego ME, Raisch DW. A literature review of cost-effectiveness analyses of prostate-specific antigen test in prostate cancer screening. Expert Review of Pharmacoeconomics & Outcomes Research. 2013;13(3):327–342. - PubMed
-
- Bradley LA, Palomaki GE, Gutman S, Samson D, Aronson N. Comparative Effectiveness Review: Prostate Cancer Antigen 3 Testing for the Diagnosis and Management of Prostate Cancer. Journal of Urology. 2013;190(2):389–398. - PubMed
-
- Snyder CF, Frick KD, Blackford AL, Herbert RJ, Neville BA, Carducci MA, Earle CC. How Does Initial Treatment Choice Affect Short-Term and Long-Term Costs for Clinically Localized Prostate Cancer? Cancer. 2010;116(23):5391–5399. - PubMed
-
- Etzioni RD. Review of evidence concerning PSA screening for prostate cancer has limitations as basis for policy development. Evidence-based medicine. 2013;18(2):75–6. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
