Studies of Hematopoietic Cell Differentiation with a Ratiometric and Reversible Sensor of Mitochondrial Reactive Oxygen Species
- PMID: 26865422
- PMCID: PMC4860629
- DOI: 10.1089/ars.2015.6495
Studies of Hematopoietic Cell Differentiation with a Ratiometric and Reversible Sensor of Mitochondrial Reactive Oxygen Species
Abstract
Aims: Chronic elevations in cellular redox state are known to result in the onset of various pathological conditions, but transient increases in reactive oxygen species (ROS)/reactive nitrogen species (RNS) are necessary for signal transduction and various physiological functions. There is a distinct lack of reversible fluorescent tools that can aid in studying and unraveling the roles of ROS/RNS in physiology and pathology by monitoring the variations in cellular ROS levels over time. In this work, we report the development of ratiometric fluorescent sensors that reversibly respond to changes in mitochondrial redox state.
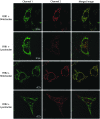
Results: Photophysical studies of the developed flavin-rhodamine redox sensors, flavin-rhodamine redox sensor 1 (FRR1) and flavin-rhodamine redox sensor 2 (FRR2), confirmed the reversible response of the probes upon reduction and re-oxidation over more than five cycles. The ratiometric output of FRR1 and FRR2 remained unaltered in the presence of other possible cellular interferants (metals and pH). Microscopy studies indicated clear mitochondrial localization of both probes, and FRR2 was shown to report the time-dependent increase of mitochondrial ROS levels after lipopolysaccharide stimulation in macrophages. Moreover, it was used to study the variations in mitochondrial redox state in mouse hematopoietic cells at different stages of embryonic development and maturation.
Innovation: This study provides the first ratiometric and reversible probes for ROS, targeted to the mitochondria, which reveal variations in mitochondrial ROS levels at different stages of embryonic and adult blood cell production.
Conclusions: Our results suggest that with their ratiometric and reversible outputs, FRR1 and FRR2 are valuable tools for the future study of oxidative stress and its implications in physiology and pathology. Antioxid. Redox Signal. 24, 667-679.
Figures









References
-
- Johnson I, Spence MTZ. (eds). Molecular Probes Handbook, A Guide to Fluorescent Probes and Labeling Technologies, 11th edn. Carlsbad, CA: Life Technologies
-
- Antas VI, Brigden KW, Prudence AJ, and Fraser ST. Gastrokine-2 is transiently expressed in the endodermal and endothelial cells of the maturing mouse yolk sac. Gene Expr Patterns 16: 69–74, 2014 - PubMed
-
- Arai Y. and Nagai T. Extensive use of FRET in biological imaging. Microscopy 62: 419–428, 2013 - PubMed
-
- Banekovich C. and Matuszczak B. 2′,3′,4′,5′-Tetraacetyl-N(3)-carboxymethylriboflavin derivatives: synthesis and fluorescence studies. Tetrahedron Lett 46: 5053–5056, 2005
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
