Scavenger receptor B1, the HDL receptor, is expressed abundantly in liver sinusoidal endothelial cells
- PMID: 26865459
- PMCID: PMC4749959
- DOI: 10.1038/srep20646
Scavenger receptor B1, the HDL receptor, is expressed abundantly in liver sinusoidal endothelial cells
Abstract
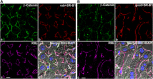
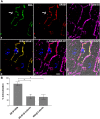
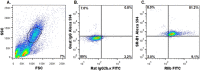
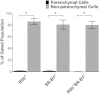
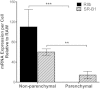
Cholesterol from peripheral tissue, carried by HDL, is metabolized in the liver after uptake by the HDL receptor, SR-B1. Hepatocytes have long been considered the only liver cells expressing SR-B1; however, in this study we describe two disparate immunofluorescence (IF) experiments that suggest otherwise. Using high-resolution confocal microscopy employing ultrathin (120 nm) sections of mouse liver, improving z-axis resolution, we identified the liver sinusoidal endothelial cells (LSEC), marked by FcγRIIb, as the cell within the liver expressing abundant SR-B1. In contrast, the hepatocyte, identified with β-catenin, expressed considerably weaker levels, although optical resolution of SR-B1 was inadequate. Thus, we moved to a different IF strategy, first separating dissociated liver cells by gradient centrifugation into two portions, hepatocytes (parenchymal cells) and LSEC (non-parenchymal cells). Characterizing both portions for the cellular expression of SR-B1 by flow cytometry, we found that LSEC expressed considerable amounts of SR-B1 while in hepatocytes SR-B1 expression was barely perceptible. Assessing mRNA of SR-B1 by real time PCR we found messenger expression in LSEC to be about 5 times higher than in hepatocytes.
Figures



Similar articles
-
Chronic Moderate Alcohol Intakes Accelerate SR-B1 Mediated Reverse Cholesterol Transport.Sci Rep. 2016 Sep 13;6:33032. doi: 10.1038/srep33032. Sci Rep. 2016. PMID: 27618957 Free PMC article.
-
The role of human and mouse hepatic scavenger receptor class B type I (SR-BI) in the selective uptake of low-density lipoprotein-cholesteryl esters.Biochemistry. 2003 Jun 24;42(24):7527-38. doi: 10.1021/bi026949a. Biochemistry. 2003. PMID: 12809509
-
Human scavenger receptor class B type I is expressed with cell-specific fashion in both initial and terminal site of reverse cholesterol transport.Atherosclerosis. 2005 Nov;183(1):75-83. doi: 10.1016/j.atherosclerosis.2005.02.035. Epub 2005 Jun 20. Atherosclerosis. 2005. PMID: 15970294
-
Macrophage SR-B1 in atherosclerotic cardiovascular disease.Curr Opin Lipidol. 2022 Jun 1;33(3):167-174. doi: 10.1097/MOL.0000000000000822. Epub 2022 Mar 7. Curr Opin Lipidol. 2022. PMID: 35258032 Review.
-
Prominent Receptors of Liver Sinusoidal Endothelial Cells in Liver Homeostasis and Disease.Front Physiol. 2020 Jul 21;11:873. doi: 10.3389/fphys.2020.00873. eCollection 2020. Front Physiol. 2020. PMID: 32848838 Free PMC article. Review.
Cited by
-
Blood-Borne Lipopolysaccharide Is Rapidly Eliminated by Liver Sinusoidal Endothelial Cells via High-Density Lipoprotein.J Immunol. 2016 Sep 15;197(6):2390-9. doi: 10.4049/jimmunol.1600702. Epub 2016 Aug 17. J Immunol. 2016. PMID: 27534554 Free PMC article.
-
The gut-liver axis in sepsis: interaction mechanisms and therapeutic potential.Crit Care. 2022 Jul 13;26(1):213. doi: 10.1186/s13054-022-04090-1. Crit Care. 2022. PMID: 35831877 Free PMC article. Review.
-
Binding of Nanoparticles Harboring Recombinant Large Surface Protein of Hepatitis B Virus to Scavenger Receptor Class B Type 1.Viruses. 2021 Jul 10;13(7):1334. doi: 10.3390/v13071334. Viruses. 2021. PMID: 34372540 Free PMC article.
-
Research Progress on the Immune Function of Liver Sinusoidal Endothelial Cells in Sepsis.Cells. 2025 Mar 4;14(5):373. doi: 10.3390/cells14050373. Cells. 2025. PMID: 40072101 Free PMC article. Review.
-
The Endothelium Is Both a Target and a Barrier of HDL's Protective Functions.Cells. 2021 Apr 28;10(5):1041. doi: 10.3390/cells10051041. Cells. 2021. PMID: 33924941 Free PMC article. Review.
References
-
- Miller N. E. & Miller G. J. Letter: High-density lipoprotein and atherosclerosis. Lancet 1, 1033 (1975). - PubMed
-
- Krieger M. Charting the fate of the “good cholesterol”: identification and characterization of the high-density lipoprotein receptor SR-BI. Annu. Rev. Biochem. 68, 523–558 (1999). - PubMed
-
- Trigatti B. L., Krieger M. & Rigotti A. Influence of the HDL receptor SR-BI on lipoprotein metabolism and atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 23, 1732–1738 (2003). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

