Increased Protein Stability and Decreased Protein Turnover in the Caenorhabditis elegans Ins/IGF-1 daf-2 Mutant
- PMID: 26865495
- PMCID: PMC5106850
- DOI: 10.1093/gerona/glv221
Increased Protein Stability and Decreased Protein Turnover in the Caenorhabditis elegans Ins/IGF-1 daf-2 Mutant
Abstract
In Caenorhabditis elegans, cellular proteostasis is likely essential for longevity. Autophagy has been shown to be essential for lifespan extension of daf-2 insulin/IGF mutants. Therefore, it can be hypothesized that daf-2 mutants achieve this phenotype by increasing protein turnover. However, such a mechanism would exert a substantial energy cost. By using classical 35S pulse-chase labeling, we observed that protein synthesis and degradation rates are decreased in young adults of the daf-2 insulin/IGF mutants. Although reduction of protein turnover may be energetically favorable, it may lead to accumulation and aggregation of damaged proteins. As this has been shown not to be the case in daf-2 mutants, another mechanism must exist to maintain proteostasis in this strain. We observed that proteins isolated from daf-2 mutants are more soluble in acidic conditions due to increased levels of trehalose. This suggests that trehalose may decrease the potential for protein aggregation and increases proteostasis in the daf-2 mutants. We postulate that daf-2 mutants save energy by decreasing protein turnover rates and instead stabilize their proteome by trehalose.
Keywords: Caenorhabditis; Protein metabolism; Radiolabeling.; Trehalose.
© The Author 2016. Published by Oxford University Press on behalf of The Gerontological Society of America.
Figures
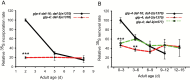
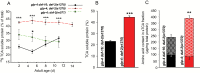
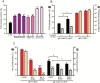
References
-
- Van Remmen H, Ward W, Sabia R, Richardson A. Gene expression and protein degradation. In: Masoro EJ, eds. Handbook of Physiology: Section 11: Aging. Oxford, UK: Oxford University Press; 1995:71–234.
-
- Rattan SI. Synthesis, modifications, and turnover of proteins during aging. Exp Gerontol. 1996;31:33–47. doi:10.1016/0531-5565(95)02022-5 - PubMed
-
- Hipkiss AR. Accumulation of altered proteins and ageing: causes and effects. Exp Gerontol. 2006;41:464–473. doi:10.1016/j.exger.2006.03.004 - PubMed
-
- Lindner AB, Demarez A. Protein aggregation as a paradigm of aging. Biochim Biophys Acta. 2009;1790:980–996. doi:10.1016/j.bbagen.2009.06.005 - PubMed
-
- Carrard G, Bulteau AL, Petropoulos I, Friguet B. Impairment of proteasome structure and function in aging. Int J Biochem Cell Biol. 2002;34:1461–1474. doi:10.1016/S1357-2725(02)00085-7 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

