Pectic polysaccharides are attacked by hydroxyl radicals in ripening fruit: evidence from a fluorescent fingerprinting method
- PMID: 26865506
- PMCID: PMC4765547
- DOI: 10.1093/aob/mcv192
Pectic polysaccharides are attacked by hydroxyl radicals in ripening fruit: evidence from a fluorescent fingerprinting method
Abstract
Background and aims: Many fruits soften during ripening, which is important commercially and in rendering the fruit attractive to seed-dispersing animals. Cell-wall polysaccharide hydrolases may contribute to softening, but sometimes appear to be absent. An alternative hypothesis is that hydroxyl radicals ((•)OH) non-enzymically cleave wall polysaccharides. We evaluated this hypothesis by using a new fluorescent labelling procedure to 'fingerprint' (•)OH-attacked polysaccharides.
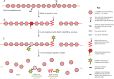
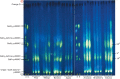
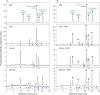
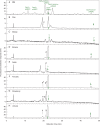
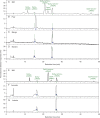
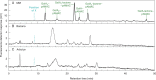
Methods: We tagged fruit polysaccharides with 2-(isopropylamino)-acridone (pAMAC) groups to detect (a) any mid-chain glycosulose residues formed in vivo during (•)OH action and (b) the conventional reducing termini. The pAMAC-labelled pectins were digested with Driselase, and the products resolved by high-voltage electrophoresis and high-pressure liquid chromatography.
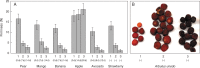
Key results: Strawberry, pear, mango, banana, apple, avocado, Arbutus unedo, plum and nectarine pectins all yielded several pAMAC-labelled products. GalA-pAMAC (monomeric galacturonate, labelled with pAMAC at carbon-1) was produced in all species, usually increasing during fruit softening. The six true fruits also gave pAMAC·UA-GalA disaccharides (where pAMAC·UA is an unspecified uronate, labelled at a position other than carbon-1), with yields increasing during softening. Among false fruits, apple and strawberry gave little pAMAC·UA-GalA; pear produced it transiently.
Conclusions: GalA-pAMAC arises from pectic reducing termini, formed by any of three proposed chain-cleaving agents ((•)OH, endopolygalacturonase and pectate lyase), any of which could cause its ripening-related increase. In contrast, pAMAC·UA-GalA conjugates are diagnostic of mid-chain oxidation of pectins by (•)OH. The evidence shows that (•)OH radicals do indeed attack fruit cell wall polysaccharides non-enzymically during softening in vivo. This applies much more prominently to drupes and berries (true fruits) than to false fruits (swollen receptacles). (•)OH radical attack on polysaccharides is thus predominantly a feature of ovary-wall tissue.
Keywords: Fruit; cell wall; fingerprint compounds; fluorescent labelling; hydroxyl radicals; non-enzymic scission; pectic polysaccharides; ripening.
© The Author 2016. Published by Oxford University Press on behalf of the Annals of Botany Company. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures
References
-
- Albersheim P, Darvill A, Roberts K, Sederoff R, Staehelin A. 2010. Plant cell walls. From chemistry to biology. New York: Garland Science.
-
- de Alcântara PH, Martim L, Silva CO, Dietrich SM, Buckeridge MS. 2006. Purification of a β-galactosidase from cotyledons of Hymenaea courbaril L. (Leguminosae). Enzyme properties and biological function. Plant Physiology and Biochemistry 44: 619–627. - PubMed
-
- Ali ZM, Chin LH, Lazan H. 2004. A comparative study on wall degrading enzymes, pectin modifications and softening during ripening of selected tropical fruits. Plant Science 167: 317–327.
-
- Asthir B, Duffus CM, Smith RC, Spoor W. 2002. Diamine oxidase is involved in H2O2 production in the chalazal cells during barley grain filling. Journal of Experimental Botany 53: 677–682. - PubMed
-
- Basanta MF, Ponce NM, Salum ML, et al. 2014. Compositional changes in cell wall polysaccharides from five sweet cherry (Prunus avium L.) cultivars during on-tree ripening. Journal of Agricultural and Food Chemistry 62: 12418–12427. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

