Results from a phase 1 study of nusinersen (ISIS-SMN(Rx)) in children with spinal muscular atrophy
- PMID: 26865511
- PMCID: PMC4782111
- DOI: 10.1212/WNL.0000000000002445
Results from a phase 1 study of nusinersen (ISIS-SMN(Rx)) in children with spinal muscular atrophy
Abstract
Objective: To examine safety, tolerability, pharmacokinetics, and preliminary clinical efficacy of intrathecal nusinersen (previously ISIS-SMNRx), an antisense oligonucleotide designed to alter splicing of SMN2 mRNA, in patients with childhood spinal muscular atrophy (SMA).
Methods: Nusinersen was delivered by intrathecal injection to medically stable patients with type 2 and type 3 SMA aged 2-14 years in an open-label phase 1 study and its long-term extension. Four ascending single-dose levels (1, 3, 6, and 9 mg) were examined in cohorts of 6-10 participants. Participants were monitored for safety and tolerability, and CSF and plasma pharmacokinetics were measured. Exploratory efficacy endpoints included the Hammersmith Functional Motor Scale Expanded (HFMSE) and Pediatric Quality of Life Inventory.
Results: A total of 28 participants enrolled in the study (n = 6 in first 3 dose cohorts; n = 10 in the 9-mg cohort). Intrathecal nusinersen was well-tolerated with no safety/tolerability concerns identified. Plasma and CSF drug levels were dose-dependent, consistent with preclinical data. Extended pharmacokinetics indicated a prolonged CSF drug half-life of 4-6 months after initial clearance. A significant increase in HFMSE scores was observed at the 9-mg dose at 3 months postdose (3.1 points; p = 0.016), which was further increased 9-14 months postdose (5.8 points; p = 0.008) during the extension study.
Conclusions: Results from this study support continued development of nusinersen for treatment of SMA.
Classification of evidence: This study provides Class IV evidence that in children with SMA, intrathecal nusinersen is not associated with safety or tolerability concerns.
© 2016 American Academy of Neurology.
Figures
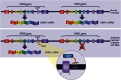
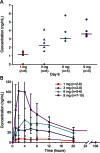
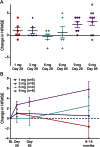
Comment in
-
Spinal muscular atrophy: A preliminary result toward new therapy.Neurology. 2016 Mar 8;86(10):884-5. doi: 10.1212/WNL.0000000000002453. Epub 2016 Feb 10. Neurology. 2016. PMID: 26865521 No abstract available.
References
-
- Lunn MR, Wang CH. Spinal muscular atrophy. Lancet 2008;371:2120–2133. - PubMed
-
- Darras BT, Markowitz JA, Monani UR, De Vivo DC. Spinal muscular atrophies. In: Darras BT, Jones HR, Jr, Ryan MM, De Vivo DC, eds. Neuromuscular Disorders of Infancy, Childhood, and Adolescence: A Clinician's Approach, 2nd ed San Diego: Academic Press; 2014:117–145.
-
- Russman BS. Spinal muscular atrophy: clinical classification and disease heterogeneity. J Child Neurol 2007;22:946–951. - PubMed
-
- Lefebvre S, Bürglen L, Reboullet S, et al. Identification and characterization of a spinal muscular atrophy-determining gene. Cell 1995;80:1155–1165. - PubMed
-
- Cartegni L, Krainer AR. Disruption of an SF2/ASF-dependent exonic splicing enhancer in SMN2 causes spinal muscular atrophy in the absence of SMN1. Nat Genet 2002;30:377–384. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
