The Next Generation of Platinum Drugs: Targeted Pt(II) Agents, Nanoparticle Delivery, and Pt(IV) Prodrugs
- PMID: 26865551
- PMCID: PMC4792284
- DOI: 10.1021/acs.chemrev.5b00597
The Next Generation of Platinum Drugs: Targeted Pt(II) Agents, Nanoparticle Delivery, and Pt(IV) Prodrugs
Abstract
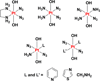
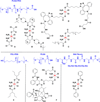
The platinum drugs, cisplatin, carboplatin, and oxaliplatin, prevail in the treatment of cancer, but new platinum agents have been very slow to enter the clinic. Recently, however, there has been a surge of activity, based on a great deal of mechanistic information, aimed at developing nonclassical platinum complexes that operate via mechanisms of action distinct from those of the approved drugs. The use of nanodelivery devices has also grown, and many different strategies have been explored to incorporate platinum warheads into nanomedicine constructs. In this Review, we discuss these efforts to create the next generation of platinum anticancer drugs. The introduction provides the reader with a brief overview of the use, development, and mechanism of action of the approved platinum drugs to provide the context in which more recent research has flourished. We then describe approaches that explore nonclassical platinum(II) complexes with trans geometry or with a monofunctional coordination mode, polynuclear platinum(II) compounds, platinum(IV) prodrugs, dual-threat agents, and photoactivatable platinum(IV) complexes. Nanoparticles designed to deliver platinum(IV) complexes will also be discussed, including carbon nanotubes, carbon nanoparticles, gold nanoparticles, quantum dots, upconversion nanoparticles, and polymeric micelles. Additional nanoformulations, including supramolecular self-assembled structures, proteins, peptides, metal-organic frameworks, and coordination polymers, will then be described. Finally, the significant clinical progress made by nanoparticle formulations of platinum(II) agents will be reviewed. We anticipate that such a synthesis of disparate research efforts will not only help to generate new drug development ideas and strategies, but also will reflect our optimism that the next generation of approved platinum cancer drugs is about to arrive.
Figures























References
-
- Howlader N, Noone AM, Krapcho M, Neyman N, Aminou R, Waldron W, Altekruse SF, Kosary CL, Ruhl J, Tatalovich Z, Cho H, Mariotto A, Eisner MP, Lewis DR, Chen HS, Feuer EJ, Cronin KA. Bethesda, MD: National Cancer Institute; 2012.
-
- World Health Organization. WHO Model List of Essential Medicines. 2013
-
- Centers for Disease Control and Prevention. [Accessed: 2015];Ambulatory Care Drug Database System. http://www.cdc.gov/nchs/ahcd/ahcd_database.htm.
-
- National Institutes of Health. [Accessed: 2015]; www.clinicaltrials.gov.
-
- Haug C, Gøtzsche PC, Schroeder TV. N Engl. J. Med. 2005;353:2811–2812. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

