Filaggrin inhibits generation of CD1a neolipid antigens by house dust mite-derived phospholipase
- PMID: 26865566
- PMCID: PMC4872823
- DOI: 10.1126/scitranslmed.aad6833
Filaggrin inhibits generation of CD1a neolipid antigens by house dust mite-derived phospholipase
Abstract
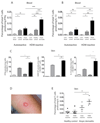
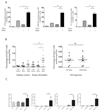
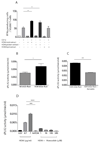
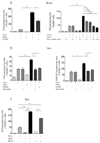
Atopic dermatitis is a common pruritic skin disease in which barrier dysfunction and cutaneous inflammation contribute to pathogenesis. Mechanisms underlying the associated inflammation are not fully understood, and although Langerhans cells expressing the nonclassical major histocompatibility complex (MHC) family member CD1a are known to be enriched within lesions, their role in clinical disease pathogenesis has not been studied. We observed that house dust mite (HDM) allergen generates neolipid antigens presented by CD1a to T cells in the blood and skin lesions of affected individuals. HDM-responsive CD1a-reactive T cells increased in frequency after birth in individuals with atopic dermatitis and showed rapid effector function, consistent with antigen-driven maturation. In HDM-challenged human skin, we observed phospholipase A2 (PLA2) activity in vivo. CD1a-reactive T cell activation was dependent on HDM-derived PLA2, and such cells infiltrated the skin after allergen challenge. Moreover, we observed that the skin barrier protein filaggrin, insufficiency of which is associated with atopic skin disease, inhibited PLA2 activity and decreased CD1a-reactive PLA2-generated neolipid-specific T cell activity from skin and blood. The most widely used classification schemes of hypersensitivity suggest that nonpeptide stimulants of T cells act as haptens that modify peptides or proteins; however, our results show that HDM proteins may also generate neolipid antigens that directly activate T cells. These data define PLA2 inhibition as a function of filaggrin, supporting PLA2 inhibition as a therapeutic approach.
Copyright © 2016, American Association for the Advancement of Science.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Asher MI, Montefort S, Bjorksten B, Lai CK, Strachan DP, Weiland SK, Williams H. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet. 2006;368:733–743. - PubMed
-
- Palmer CN, Irvine AD, Terron-Kwiatkowski A, Zhao Y, Liao H, Lee SP, Goudie DR, Sandilands A, Campbell LE, Smith FJ, O'Regan GM, et al. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat Genet. 2006;38:441–446. - PubMed
-
- Rodríguez E, Baurecht H, Herberich E, Wagenpfeil S, Brown SJ, Cordell HJ, Irvine AD, Weidinger S. Meta-analysis of filaggrin polymorphisms in eczema and asthma: Robust risk factors in atopic disease. Journal of Allergy and Clinical Immunology. 2009;123:1361–1370.e1367. - PubMed
-
- Sandford AJ, Shirakawa T, Moffatt MF, Daniels SE, Ra C, Faux JA, Young RP, Nakamura Y, Lathrop GM, Cookson WO, et al. Localisation of atopy and beta subunit of high-affinity IgE receptor (Fc epsilon RI) on chromosome 11q. Lancet. 1993;341:332–334. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

