Humoral and Cell-Mediated Immune Responses to Alternate Booster Schedules of Anthrax Vaccine Adsorbed in Humans
- PMID: 26865594
- PMCID: PMC4820509
- DOI: 10.1128/CVI.00696-15
Humoral and Cell-Mediated Immune Responses to Alternate Booster Schedules of Anthrax Vaccine Adsorbed in Humans
Abstract
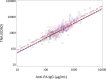
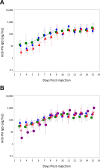
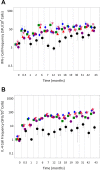
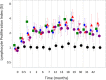
Protective antigen (PA)-specific antibody and cell-mediated immune (CMI) responses to annual and alternate booster schedules of anthrax vaccine adsorbed (AVA; BioThrax) were characterized in humans over 43 months. Study participants received 1 of 6 vaccination schedules: a 3-dose intramuscular (IM) priming series (0, 1, and 6 months) with a single booster at 42 months (4-IM); 3-dose IM priming with boosters at 18 and 42 months (5-IM); 3-dose IM priming with boosters at 12, 18, 30, and 42 months (7-IM); the 1970 licensed priming series of 6 doses (0, 0.5, 1, 6, 12, and 18 months) and two annual boosters (30 and 42 months) administered either subcutaneously (SQ) (8-SQ) or IM (8-IM); or saline placebo control at all eight time points. Antibody response profiles included serum anti-PA IgG levels, subclass distributions, avidity, and lethal toxin neutralization activity (TNA). CMI profiles included frequencies of gamma interferon (IFN-γ)- and interleukin 4 (IL-4)-secreting cells and memory B cells (MBCs), lymphocyte stimulation indices (SI), and induction of IFN-γ, IL-2, IL-4, IL-6, IL-1β, and tumor necrosis factor alpha (TNF-α) mRNA. All active schedules elicited high-avidity PA-specific IgG, TNA, MBCs, and T cell responses with a mixed Th1-Th2 profile and Th2 dominance. Anti-PA IgG and TNA were highly correlated (e.g., month 7,r(2)= 0.86,P< 0.0001, log10 transformed) and declined in the absence of boosters. Boosters administered IM generated the highest antibody responses. Increasing time intervals between boosters generated antibody responses that were faster than and superior to those obtained with the final month 42 vaccination. CMI responses to the 3-dose IM priming remained elevated up to 43 months. (This study has been registered at ClinicalTrials.gov under registration no. NCT00119067.).
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures


References
-
- Marano N, Plikaytis BD, Martin SW, Rose C, Semenova VA, Martin SK, Freeman AE, Li H, Mulligan MJ, Parker SD, Babcock J, Keitel W, El Sahly H, Poland GA, Jacobson RM, Keyserling HL, Soroka SD, Fox SP, Stamper JL, McNeil MM, Perkins BA, Messonnier N, Quinn CP. 2008. Effects of a reduced dose schedule and intramuscular administration of anthrax vaccine adsorbed on immunogenicity and safety at 7 months: a randomized trial. JAMA 300:1532–1543. doi:10.1001/jama.300.13.1532. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

