miR-124 Regulates the Phase of Drosophila Circadian Locomotor Behavior
- PMID: 26865623
- PMCID: PMC4748081
- DOI: 10.1523/JNEUROSCI.3286-15.2016
miR-124 Regulates the Phase of Drosophila Circadian Locomotor Behavior
Abstract
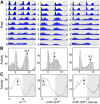
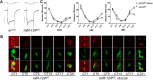
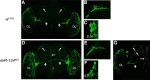
Animals use circadian rhythms to anticipate daily environmental changes. Circadian clocks have a profound effect on behavior. In Drosophila, for example, brain pacemaker neurons dictate that flies are mostly active at dawn and dusk. miRNAs are small, regulatory RNAs (≈22 nt) that play important roles in posttranscriptional regulation. Here, we identify miR-124 as an important regulator of Drosophila circadian locomotor rhythms. Under constant darkness, flies lacking miR-124 (miR-124(KO)) have a dramatically advanced circadian behavior phase. However, whereas a phase defect is usually caused by a change in the period of the circadian pacemaker, this is not the case in miR-124(KO) flies. Moreover, the phase of the circadian pacemaker in the clock neurons that control rhythmic locomotion is not altered either. Therefore, miR-124 modulates the output of circadian clock neurons rather than controlling their molecular pacemaker. Circadian phase is also advanced under temperature cycles, but a light/dark cycle partially corrects the defects in miR-124(KO) flies. Indeed, miR-124(KO) shows a normal evening phase under the latter conditions, but morning behavioral activity is suppressed. In summary, miR-124 controls diurnal activity and determines the phase of circadian locomotor behavior without affecting circadian pacemaker function. It thus provides a potent entry point to elucidate the mechanisms by which the phase of circadian behavior is determined.
Significance statement: In animals, molecular circadian clocks control the timing of behavioral activities to optimize them with the day/night cycle. This is critical for their fitness and survival. The mechanisms by which the phase of circadian behaviors is determined downstream of the molecular pacemakers are not yet well understood. Recent studies indicate that miRNAs are important regulators of circadian outputs. We found that miR-124 shapes diurnal behavioral activity and has a striking impact on the phase of circadian locomotor behavior. Surprisingly, the period and phase of the neural circadian pacemakers driving locomotor rhythms are unaffected. Therefore, miR-124 is a critical modulator of the circadian output pathways that control circadian behavioral rhythms.
Keywords: Drosophila; circadian behavior; circadian rhythms; miRNAs.
Copyright © 2016 the authors 0270-6474/16/362007-07$15.00/0.
Figures

Similar articles
-
miR-124 Regulates Diverse Aspects of Rhythmic Behavior in Drosophila.J Neurosci. 2016 Mar 23;36(12):3414-21. doi: 10.1523/JNEUROSCI.3287-15.2016. J Neurosci. 2016. PMID: 27013671 Free PMC article.
-
miR-210 controls the evening phase of circadian locomotor rhythms through repression of Fasciclin 2.PLoS Genet. 2019 Jul 29;15(7):e1007655. doi: 10.1371/journal.pgen.1007655. eCollection 2019 Jul. PLoS Genet. 2019. PMID: 31356596 Free PMC article.
-
The Drosophila Receptor Protein Tyrosine Phosphatase LAR Is Required for Development of Circadian Pacemaker Neuron Processes That Support Rhythmic Activity in Constant Darkness But Not during Light/Dark Cycles.J Neurosci. 2016 Mar 30;36(13):3860-70. doi: 10.1523/JNEUROSCI.4523-15.2016. J Neurosci. 2016. PMID: 27030770 Free PMC article.
-
Neural circuits underlying circadian behavior in Drosophila melanogaster.Behav Processes. 2006 Feb 28;71(2-3):211-25. doi: 10.1016/j.beproc.2005.12.008. Epub 2006 Jan 18. Behav Processes. 2006. PMID: 16414209 Review.
-
Circadian Rhythms and Sleep in Drosophila melanogaster.Genetics. 2017 Apr;205(4):1373-1397. doi: 10.1534/genetics.115.185157. Genetics. 2017. PMID: 28360128 Free PMC article. Review.
Cited by
-
Astrocytic GABA transporter controls sleep by modulating GABAergic signaling in Drosophila circadian neurons.Curr Biol. 2022 May 9;32(9):1895-1908.e5. doi: 10.1016/j.cub.2022.02.066. Epub 2022 Mar 17. Curr Biol. 2022. PMID: 35303417 Free PMC article.
-
Molecular modulators of the circadian clock: lessons from flies and mice.Cell Mol Life Sci. 2017 Mar;74(6):1035-1059. doi: 10.1007/s00018-016-2378-8. Epub 2016 Sep 29. Cell Mol Life Sci. 2017. PMID: 27689221 Free PMC article. Review.
-
Dopaminergic Ric GTPase activity impacts amphetamine sensitivity and sleep quality in a dopamine transporter-dependent manner in Drosophila melanogaster.Mol Psychiatry. 2021 Dec;26(12):7793-7802. doi: 10.1038/s41380-021-01275-y. Epub 2021 Sep 1. Mol Psychiatry. 2021. PMID: 34471250 Free PMC article.
-
Pervasive Behavioral Effects of MicroRNA Regulation in Drosophila.Genetics. 2017 Jul;206(3):1535-1548. doi: 10.1534/genetics.116.195776. Epub 2017 May 3. Genetics. 2017. PMID: 28468905 Free PMC article.
-
Regulation of circadian rhythm and sleep by miR-375-timeless interaction in Drosophila.FASEB J. 2020 Dec;34(12):16536-16551. doi: 10.1096/fj.202001107R. Epub 2020 Oct 20. FASEB J. 2020. PMID: 33078445 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
