Evidence That the Laminar Fate of LGE/CGE-Derived Neocortical Interneurons Is Dependent on Their Progenitor Domains
- PMID: 26865626
- PMCID: PMC6602017
- DOI: 10.1523/JNEUROSCI.3550-15.2016
Evidence That the Laminar Fate of LGE/CGE-Derived Neocortical Interneurons Is Dependent on Their Progenitor Domains
Abstract
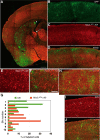
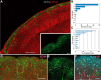
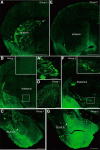
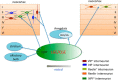
Neocortical interneurons show tremendous diversity in terms of their neurochemical marker expressions, morphology, electrophysiological properties, and laminar fate. Allocation of interneurons to their appropriate regions and layers in the neocortex is thought to play important roles for the emergence of higher functions of the neocortex. Neocortical interneurons mainly originate from the medial ganglionic eminence (MGE) and the caudal ganglionic eminence (CGE). The diversity and the laminar fate of MGE-derived interneurons depend on the location of their birth and birthdate, respectively. However, this relationship does not hold for CGE-derived interneurons. Here, using the method of in utero electroporation, which causes arbitrary occurrence of labeled progenitor domains, we tracked all descendants of the lateral ganglionic eminence (LGE)/CGE progenitors in mice. We provide evidence that neocortical interneurons with distinct laminar fate originate from distinct progenitor domains within the LGE/CGE. We find layer I interneurons are predominantly labeled in a set of animals, whereas other upper layer neurons are predominantly labeled in another set. We also find distinct subcortical structures labeled between the two sets. Further, interneurons labeled in layer I show distinct neurochemical properties from those in other layers. Together, these results suggest that the laminar fate of LGE/CGE-derived interneurons depends on their spatial origin.
Significance statement: Diverse types of neocortical interneurons have distinct laminar fate, neurochemical marker expression, morphology, and electrophysiological properties. Although the specifications and laminar fate of medial ganglionic eminence-derived neocortical interneurons depend on their location of embryonic origin and birthdate, no similar causality of lateral/caudal ganglionic eminence (LGE/CGE)-derived neocortical interneurons is known. Here, we performed in utero electroporation on mouse LGE/CGE and found two groups of animals, one with preferential labeling of layer I and the other with preferential labeling of other layers. Interneurons labeled in these two groups show distinct neurochemical properties and morphologies and are associated with labeling of distinct subcortical structures. These findings suggest that the laminar fate of LGE/CGE-derived neocortical interneurons depends on their spatial origin.
Keywords: caudal ganglionic eminence; cortex; in utero electroporation; interneuron; laminar fate.
Copyright © 2016 the authors 0270-6474/16/362044-13$15.00/0.
Figures




Similar articles
-
Nuclear receptor COUP-TFII-expressing neocortical interneurons are derived from the medial and lateral/caudal ganglionic eminence and define specific subsets of mature interneurons.J Comp Neurol. 2013 Feb 1;521(2):479-97. doi: 10.1002/cne.23186. J Comp Neurol. 2013. PMID: 22791192
-
Transcriptional Regulation of Cortical Interneuron Development.In: Noebels JL, Avoli M, Rogawski MA, Vezzani A, Delgado-Escueta AV, editors. Jasper's Basic Mechanisms of the Epilepsies. 5th edition. New York: Oxford University Press; 2024. Chapter 47. In: Noebels JL, Avoli M, Rogawski MA, Vezzani A, Delgado-Escueta AV, editors. Jasper's Basic Mechanisms of the Epilepsies. 5th edition. New York: Oxford University Press; 2024. Chapter 47. PMID: 39637134 Free Books & Documents. Review.
-
The germinal zones of the basal ganglia but not the septum generate GABAergic interneurons for the cortex.J Neurosci. 2010 Sep 8;30(36):12050-62. doi: 10.1523/JNEUROSCI.6178-09.2010. J Neurosci. 2010. PMID: 20826668 Free PMC article.
-
Islet1 Precursors Contribute to Mature Interneuron Subtypes in Mouse Neocortex.Cereb Cortex. 2021 Oct 1;31(11):5206-5224. doi: 10.1093/cercor/bhab152. Cereb Cortex. 2021. PMID: 34228108 Free PMC article.
-
Elucidating the developmental trajectories of GABAergic cortical interneuron subtypes.Neurosci Res. 2019 Jan;138:26-32. doi: 10.1016/j.neures.2018.09.012. Epub 2018 Sep 15. Neurosci Res. 2019. PMID: 30227162 Review.
Cited by
-
Differential potentials of neural progenitors for the generation of neurons and non-neuronal cells in the developing amniote brain.Sci Rep. 2019 Mar 14;9(1):4514. doi: 10.1038/s41598-019-40599-2. Sci Rep. 2019. PMID: 30872629 Free PMC article.
-
Rostro-Caudal and Caudo-Rostral Migrations in the Telencephalon: Going Forward or Backward?Front Neurosci. 2017 Dec 21;11:692. doi: 10.3389/fnins.2017.00692. eCollection 2017. Front Neurosci. 2017. PMID: 29311773 Free PMC article. Review.
-
Gene-environment interactions in cortical interneuron development and dysfunction: A review of preclinical studies.Neurotoxicology. 2017 Jan;58:120-129. doi: 10.1016/j.neuro.2016.12.002. Epub 2016 Dec 5. Neurotoxicology. 2017. PMID: 27932026 Free PMC article. Review.
-
Cross-species single-cell transcriptomics reveals neuronal similarities and heterogeneity in amniote pallium.Zool Res. 2025 Jan 18;46(1):193-208. doi: 10.24272/j.issn.2095-8137.2024.102. Zool Res. 2025. PMID: 39846196 Free PMC article.
-
Cortical distribution of GABAergic interneurons is determined by migration time and brain size.Development. 2020 Jul 22;147(14):dev185033. doi: 10.1242/dev.185033. Development. 2020. PMID: 32586977 Free PMC article.
References
-
- Altman J, Bayer SA. Time of origin of neurons of the rat superior colliculus in relation to other components of the visual and visuomotor pathways. Exp Brain Res. 1981;42:424–434. - PubMed
-
- Anderson SA, Marín O, Horn C, Jennings K, Rubenstein JL. Distinct cortical migrations from the medial and lateral ganglionic eminences. Development. 2001;128:353–363. - PubMed
-
- Angevine JB, Jr, Sidman RL. Autoradiographic study of cell migration during histogenesis of cerebral cortex in the mouse. Nature. 1961;192:766–768. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
