Cholinergic Neurons in the Basal Forebrain Promote Wakefulness by Actions on Neighboring Non-Cholinergic Neurons: An Opto-Dialysis Study
- PMID: 26865627
- PMCID: PMC4748083
- DOI: 10.1523/JNEUROSCI.3318-15.2016
Cholinergic Neurons in the Basal Forebrain Promote Wakefulness by Actions on Neighboring Non-Cholinergic Neurons: An Opto-Dialysis Study
Abstract
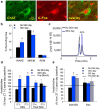
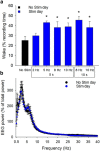
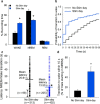
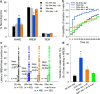
Understanding the control of sleep-wake states by the basal forebrain (BF) poses a challenge due to the intermingled presence of cholinergic, GABAergic, and glutamatergic neurons. All three BF neuronal subtypes project to the cortex and are implicated in cortical arousal and sleep-wake control. Thus, nonspecific stimulation or inhibition studies do not reveal the roles of these different neuronal types. Recent studies using optogenetics have shown that "selective" stimulation of BF cholinergic neurons increases transitions between NREM sleep and wakefulness, implicating cholinergic projections to cortex in wake promotion. However, the interpretation of these optogenetic experiments is complicated by interactions that may occur within the BF. For instance, a recent in vitro study from our group found that cholinergic neurons strongly excite neighboring GABAergic neurons, including the subset of cortically projecting neurons, which contain the calcium-binding protein, parvalbumin (PV) (Yang et al., 2014). Thus, the wake-promoting effect of "selective" optogenetic stimulation of BF cholinergic neurons could be mediated by local excitation of GABA/PV or other non-cholinergic BF neurons. In this study, using a newly designed opto-dialysis probe to couple selective optical stimulation with simultaneous in vivo microdialysis, we demonstrated that optical stimulation of cholinergic neurons locally increased acetylcholine levels and increased wakefulness in mice. Surprisingly, the enhanced wakefulness caused by cholinergic stimulation was abolished by simultaneous reverse microdialysis of cholinergic receptor antagonists into BF. Thus, our data suggest that the wake-promoting effect of cholinergic stimulation requires local release of acetylcholine in the basal forebrain and activation of cortically projecting, non-cholinergic neurons, including the GABAergic/PV neurons.
Significance statement: Optogenetics is a revolutionary tool to assess the roles of particular groups of neurons in behavioral functions, such as control of sleep and wakefulness. However, the interpretation of optogenetic experiments requires knowledge of the effects of stimulation on local neurotransmitter levels and effects on neighboring neurons. Here, using a novel "opto-dialysis" probe to couple optogenetics and in vivo microdialysis, we report that optical stimulation of basal forebrain (BF) cholinergic neurons in mice increases local acetylcholine levels and wakefulness. Reverse microdialysis of cholinergic antagonists within BF prevents the wake-promoting effect. This important result challenges the prevailing dictum that BF cholinergic projections to cortex directly control wakefulness and illustrates the utility of "opto-dialysis" for dissecting the complex brain circuitry underlying behavior.
Keywords: NREM to wake transitions; basal forebrain; cholinergic neurons; opto-dialysis.
Copyright © 2016 the authors 0270-6474/16/362058-11$15.00/0.
Figures

Similar articles
-
Histamine release in the basal forebrain mediates cortical activation through cholinergic neurons.J Neurosci. 2012 Sep 19;32(38):13244-54. doi: 10.1523/JNEUROSCI.5933-11.2012. J Neurosci. 2012. PMID: 22993440 Free PMC article.
-
Cholinergic neurons excite cortically projecting basal forebrain GABAergic neurons.J Neurosci. 2014 Feb 19;34(8):2832-44. doi: 10.1523/JNEUROSCI.3235-13.2014. J Neurosci. 2014. PMID: 24553925 Free PMC article.
-
Cholinergic basal forebrain structures are involved in the mediation of the arousal effect of noradrenaline.J Sleep Res. 2013 Dec;22(6):721-6. doi: 10.1111/jsr.12061. Epub 2013 May 24. J Sleep Res. 2013. PMID: 23701447
-
[Advances in the study of the effect of basal forebrain on sleep-wake regulation].Yao Xue Xue Bao. 2016 Aug;51(8):1196-201. Yao Xue Xue Bao. 2016. PMID: 29897712 Review. Chinese.
-
Magnocellular nuclei of the basal forebrain: substrates of sleep and arousal regulation.Sleep. 1995 Jul;18(6):478-500. doi: 10.1093/sleep/18.6.478. Sleep. 1995. PMID: 7481420 Review.
Cited by
-
Modulation of epileptogenesis: A paradigm for the integration of enzyme-based microelectrode arrays and optogenetics.Epilepsy Res. 2020 Jan;159:106244. doi: 10.1016/j.eplepsyres.2019.106244. Epub 2019 Nov 26. Epilepsy Res. 2020. PMID: 31816591 Free PMC article.
-
Dynamic Cholinergic Tone in the Basal Forebrain Reflects Reward-Seeking and Reinforcement During Olfactory Behavior.Front Cell Neurosci. 2021 Feb 2;15:635837. doi: 10.3389/fncel.2021.635837. eCollection 2021. Front Cell Neurosci. 2021. PMID: 33603646 Free PMC article.
-
Challenging sleep homeostasis.Neurobiol Sleep Circadian Rhythms. 2021 Jan 25;10:100060. doi: 10.1016/j.nbscr.2021.100060. eCollection 2021 May. Neurobiol Sleep Circadian Rhythms. 2021. PMID: 33604491 Free PMC article.
-
Neurobiological Underpinnings of Hyperarousal in Depression: A Comprehensive Review.Brain Sci. 2024 Jan 4;14(1):50. doi: 10.3390/brainsci14010050. Brain Sci. 2024. PMID: 38248265 Free PMC article. Review.
-
Basal Forebrain Chemogenetic Inhibition Converts the Attentional Control Mode of Goal-Trackers to That of Sign-Trackers.eNeuro. 2022 Dec 26;9(6):ENEURO.0418-22.2022. doi: 10.1523/ENEURO.0418-22.2022. Print 2022 Nov-Dec. eNeuro. 2022. PMID: 36635246 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
