Aryl Hydrocarbon Receptor Plays Protective Roles against High Fat Diet (HFD)-induced Hepatic Steatosis and the Subsequent Lipotoxicity via Direct Transcriptional Regulation of Socs3 Gene Expression
- PMID: 26865635
- PMCID: PMC4807284
- DOI: 10.1074/jbc.M115.693655
Aryl Hydrocarbon Receptor Plays Protective Roles against High Fat Diet (HFD)-induced Hepatic Steatosis and the Subsequent Lipotoxicity via Direct Transcriptional Regulation of Socs3 Gene Expression
Abstract
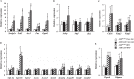
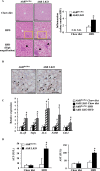
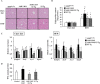
Aryl hydrocarbon receptor (AhR) is a ligand-activated transcription factor regulating the expression of genes involved in xenobiotic response. Recent studies have suggested that AhR plays essential roles not only in xenobiotic detoxification but also energy metabolism. Thus, in this study, we studied the roles of AhR in lipid metabolism. Under high fat diet (HFD) challenge, liver-specific AhR knock-out (AhR LKO) mice exhibited severe steatosis, inflammation, and injury in the liver. Gene expression analysis and biochemical study revealed thatde novolipogenesis activity was significantly increased in AhR LKO mice. In contrast, induction of suppressor of cytokine signal 3 (Socs3) expression by HFD was attenuated in the livers of AhR LKO mice. Rescue of theSocs3gene in the liver of AhR LKO mice cancelled the HFD-induced hepatic lipotoxicities. Promoter analysis established Socs3 as novel transcriptional target of AhR. These results indicated that AhR plays a protective role against HFD-induced hepatic steatosis and the subsequent lipotoxicity effects, such as inflammation, and that the mechanism of protection involves the direct transcriptional regulation ofSocs3expression by AhR.
Keywords: aryl hydrocarbon receptor (AhR); inflammation; lipid metabolism; liver; liver injury; liver metabolism; suppressor of cytokine signal 3 (Socs3).
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures






Similar articles
-
Cinnabarinic acid protects against metabolic dysfunction-associated steatohepatitis by activating aryl hydrocarbon receptor-dependent AMPK signaling.Am J Physiol Gastrointest Liver Physiol. 2025 Apr 1;328(4):G433-G447. doi: 10.1152/ajpgi.00337.2024. Epub 2025 Mar 10. Am J Physiol Gastrointest Liver Physiol. 2025. PMID: 40062565 Free PMC article.
-
Liver-specific suppressor of cytokine signaling-3 deletion in mice enhances hepatic insulin sensitivity and lipogenesis resulting in fatty liver and obesity.Hepatology. 2010 Nov;52(5):1632-42. doi: 10.1002/hep.23861. Hepatology. 2010. PMID: 20799351
-
Pdgfrα-Cre mediated knockout of the aryl hydrocarbon receptor protects mice from high-fat diet induced obesity and hepatic steatosis.PLoS One. 2020 Jul 30;15(7):e0236741. doi: 10.1371/journal.pone.0236741. eCollection 2020. PLoS One. 2020. PMID: 32730300 Free PMC article.
-
The Landscape of AhR Regulators and Coregulators to Fine-Tune AhR Functions.Int J Mol Sci. 2021 Jan 13;22(2):757. doi: 10.3390/ijms22020757. Int J Mol Sci. 2021. PMID: 33451129 Free PMC article. Review.
-
AhR and Cancer: From Gene Profiling to Targeted Therapy.Int J Mol Sci. 2021 Jan 13;22(2):752. doi: 10.3390/ijms22020752. Int J Mol Sci. 2021. PMID: 33451095 Free PMC article. Review.
Cited by
-
Role of Akkermansia muciniphila in the development of nonalcoholic fatty liver disease: current knowledge and perspectives.Front Med. 2022 Oct;16(5):667-685. doi: 10.1007/s11684-022-0960-z. Epub 2022 Nov 1. Front Med. 2022. PMID: 36318353 Review.
-
Aryl hydrocarbon receptor agonist indigo protects against obesity-related insulin resistance through modulation of intestinal and metabolic tissue immunity.Int J Obes (Lond). 2019 Dec;43(12):2407-2421. doi: 10.1038/s41366-019-0340-1. Epub 2019 Apr 3. Int J Obes (Lond). 2019. PMID: 30944419 Free PMC article.
-
Ingestion of Faecalibaculum rodentium causes depression-like phenotypes in resilient Ephx2 knock-out mice: A role of brain-gut-microbiota axis via the subdiaphragmatic vagus nerve.J Affect Disord. 2021 Sep 1;292:565-573. doi: 10.1016/j.jad.2021.06.006. Epub 2021 Jun 11. J Affect Disord. 2021. PMID: 34147969 Free PMC article.
-
Role of the Aryl Hydrocarbon Receptor (AhR) in Mediating the Effects of Coffee in the Colon.Mol Nutr Food Res. 2021 Oct;65(20):e2100539. doi: 10.1002/mnfr.202100539. Epub 2021 Aug 31. Mol Nutr Food Res. 2021. PMID: 34406707 Free PMC article.
-
Orchiectomy sensitizes cortical bone in male mice to the harmful effects of kynurenine.Bone. 2023 Aug;173:116811. doi: 10.1016/j.bone.2023.116811. Epub 2023 May 25. Bone. 2023. PMID: 37244427 Free PMC article.
References
-
- Ema M., Sogawa K., Watanabe N., Chujoh Y., Matsushita N., Gotoh O., Funae Y., and Fujii-Kuriyama Y. (1992) cDNA cloning and structure of mouse putative Ah receptor. Biochem. Biophys. Res. Commun. 184, 246–253 - PubMed
-
- Poland A., and Knutson J. C. (1982) 2,3,7,8-Tetrachlorodibenzo-p-dioxin and related halogenated aromatic hydrocarbons: examination of the mechanism of toxicity. Annu. Rev. Pharmacol. Toxicol. 22, 517–554 - PubMed
-
- Reyes H., Reisz-Porszasz S., and Hankinson O. (1992) Identification of the Ah receptor nuclear translocator protein (Arnt) as a component of the DNA binding form of the Ah receptor. Science 256, 1193–1195 - PubMed
-
- Hankinson O. (1995) The arylhydrocarbon receptor complex. Annu. Rev. Pharmacol. Toxicol. 35, 307–340 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

