Performance of the New Aptima HCV Quant Dx Assay in Comparison to the Cobas TaqMan HCV2 Test for Use with the High Pure System in Detection and Quantification of Hepatitis C Virus RNA in Plasma or Serum
- PMID: 26865682
- PMCID: PMC4809922
- DOI: 10.1128/JCM.03236-15
Performance of the New Aptima HCV Quant Dx Assay in Comparison to the Cobas TaqMan HCV2 Test for Use with the High Pure System in Detection and Quantification of Hepatitis C Virus RNA in Plasma or Serum
Abstract
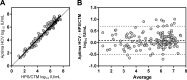
Quantitating the level of hepatitis C virus (HCV) RNA is the standard of care for monitoring HCV-infected patients during treatment. The performances of commercially available assays differ for precision, limit of detection, and limit of quantitation (LOQ). Here, we compare the performance of the Hologic Aptima HCV Quant Dx assay (Aptima) to that of the Roche Cobas TaqMan HCV test, version 2.0, using the High Pure system (HPS/CTM), considered a reference assay since it has been used in trials defining clinical decision points in patient care. The assays' performance characteristics were assessed using HCV RNA reference panels and plasma/serum from chronically HCV-infected patients. The agreement between the assays for the 3 reference panels was good, with a difference in quantitation values of <0.5 log. High concordance was demonstrated between the assays for 245 clinical samples (kappa = 0.80; 95% confidence interval [CI], 0.720 to 0.881); however, Aptima detected and/or quantitated 20 samples that HPS/CTM did not detect, while Aptima did not detect 1 sample that was quantitated by HPS/CTM. For the 165 samples quantitated by both assays, the values were highly correlated (R= 0.98;P< 0.0001). The linearity of quantitation from concentrations of 1.4 to 6 log was excellent for both assays for all HCV genotypes (GT) tested (GT 1a, 1b, 2b, and 3a) (R(2)> 0.99). The assays had similar levels of total and intra-assay variability across all genotypes at concentrations from 1,000 to 25 IU/ml. Aptima had a greater analytical sensitivity, quantitating more than 50% of replicates at 25-IU/ml target. Aptima showed performance characteristics comparable to those of HPS/CTM and increased sensitivity, making it suitable for use as a clinical diagnostic tool on the fully automated Panther platform.
Copyright © 2016 Schalasta et al.
Figures

Similar articles
-
Analytical characteristics and comparative evaluation of Aptima HCV quant Dx assay with the Abbott RealTime HCV assay and Roche COBAS AmpliPrep/COBAS TaqMan HCV quantitative test v2.0.Virol J. 2017 Apr 4;14(1):66. doi: 10.1186/s12985-017-0727-3. Virol J. 2017. PMID: 28372576 Free PMC article.
-
Analytical and clinical performance of the Hologic Aptima HCV Quant Dx Assay for the quantification of HCV RNA in plasma samples.J Virol Methods. 2017 Oct;248:159-165. doi: 10.1016/j.jviromet.2017.07.006. Epub 2017 Jul 19. J Virol Methods. 2017. PMID: 28732692
-
Evaluation of the Aptima HBV Quant assay vs. the COBAS TaqMan HBV test using the high pure system for the quantitation of HBV DNA in plasma and serum samples.Clin Chem Lab Med. 2018 Mar 28;56(4):634-641. doi: 10.1515/cclm-2017-0701. Clin Chem Lab Med. 2018. PMID: 29197859
-
Performance evaluation of the Aptima® HCV Quant Dx assay for hepatitis C virus (HCV) RNA detection and quantification in comparison to the Abbott RealTime HCV assay.J Clin Virol. 2017 Jul;92:1-6. doi: 10.1016/j.jcv.2017.04.013. Epub 2017 Apr 26. J Clin Virol. 2017. PMID: 28475925
-
Diagnostic Accuracy of Point-of-Care HCV Viral Load Assays for HCV Diagnosis: A Systematic Review and Meta-Analysis.Diagnostics (Basel). 2022 May 18;12(5):1255. doi: 10.3390/diagnostics12051255. Diagnostics (Basel). 2022. PMID: 35626411 Free PMC article. Review.
Cited by
-
Hepatitis C virus: life cycle in cells, infection and host response, and analysis of molecular markers influencing the outcome of infection and response to therapy.Clin Microbiol Infect. 2016 Oct;22(10):826-832. doi: 10.1016/j.cmi.2016.08.025. Epub 2016 Aug 31. Clin Microbiol Infect. 2016. PMID: 27592089 Free PMC article. Review.
-
Evaluation of the Aptima HCV Quant Dx Assay Using Serum and Dried Blood Spots.J Clin Microbiol. 2019 Mar 28;57(4):e00030-19. doi: 10.1128/JCM.00030-19. Print 2019 Apr. J Clin Microbiol. 2019. PMID: 30760534 Free PMC article.
-
Performance Evaluation of the Aptima Assays in Comparison with the cobas 6800 Assays for the Detection of HIV-1, HBV, and HCV in Clinical Samples.Ann Lab Med. 2022 Jul 1;42(4):447-456. doi: 10.3343/alm.2022.42.4.447. Ann Lab Med. 2022. PMID: 35177565 Free PMC article.
-
Performance Evaluation of In Vitro Screening and Diagnostic Kits for Hepatitis C Virus Infection.Front Cell Infect Microbiol. 2022 Feb 3;11:793472. doi: 10.3389/fcimb.2021.793472. eCollection 2021. Front Cell Infect Microbiol. 2022. PMID: 35186779 Free PMC article.
References
-
- World Health Organization (WHO). July 2015. Hepatitis C. Fact sheet no. 164. http://www.who.int/mediacentre/factsheets/fs164/en/. Accessed 19 November 2015.
-
- World Health Organization (WHO). 2015. Hepatitis C in the WHO European Region. Fact sheet---July 2015. http://www.euro.who.int/en/health-topics/communicable-diseases/hepatitis.... Accessed 19 November 2015.
-
- American Association for the Study of Liver Diseases (AASLD) and Infectious Diseases Society of America (IDSA). 2015. Monitoring patients who are starting hepatitis C treatment, are on treatment, or have completed therapy. In HCV guidance: recommendations for testing, managing, and treating hepatitis C. http://www.hcvguidelines.org/full-report/monitoring-patients-who-are-sta.... Accessed 11 November 2015.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

