Lymphatic Dissemination of Simian Immunodeficiency Virus after Penile Inoculation
- PMID: 26865706
- PMCID: PMC4810538
- DOI: 10.1128/JVI.02947-15
Lymphatic Dissemination of Simian Immunodeficiency Virus after Penile Inoculation
Abstract
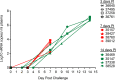
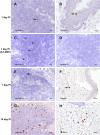
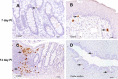
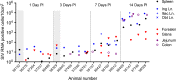
The human immunodeficiency virus (HIV) is primarily transmitted by heterosexual contact, and approximately equal numbers of men and women worldwide are infected with the virus. Understanding the biology of HIV acquisition and dissemination in men exposed to the virus by insertive penile intercourse is likely to help with the rational design of vaccines that can limit or prevent HIV transmission. To characterize the target cells and dissemination pathways involved in establishing systemic simian immunodeficiency virus (SIV) infection, we necropsied male rhesus macaques at 1, 3, 7, and 14 days after penile SIV inoculation and quantified the levels of unspliced SIV RNA and spliced SIV RNA in tissue lysates and the number of SIV RNA-positive cells in tissue sections. We found that penile (glans, foreskin, coronal sulcus) T cells and, to a lesser extent, macrophages and dendritic cells are primary targets of infection and that SIV rapidly reaches the regional lymph nodes. At 7 days after inoculation, SIV had disseminated to the blood, systemic lymph nodes, and mucosal lymphoid tissues. Further, at 7 days postinoculation (p.i.), spliced SIV RNA levels were the highest in the genital lymph nodes, indicating that this is the site where the infection is initially amplified. By 14 days p.i., spliced SIV RNA levels were high in all tissues, but they were the highest in the gastrointestinal tract, indicating that the primary site of virus replication had shifted from the genital lymph nodes to the gut. The stepwise pattern of virus replication and dissemination described here suggests that vaccine-elicited immune responses in the genital lymph nodes could help prevent infection after penile SIV challenge.
Importance: To be the most effective, vaccines should produce antiviral immune responses in the anatomic sites of virus replication. Thus, understanding the path taken by HIV from the mucosal surfaces, which are the site of virus exposure, to the deeper tissues where the virus replicates will provide insight into where AIDS vaccines should produce immunity to be the most effective. In this study, we determined that, by day 7 after penile inoculation, SIV has moved first to the inguinal lymph nodes and replicates to high levels. Although the virus is widely disseminated to other tissues by day 7, replication is largely limited to the inguinal lymph nodes. The step-by-step movement of SIV from penile mucosal surfaces to the draining lymph nodes may allow an HIV vaccine that produces immunity in these lymph nodes to block HIV from establishing an infection in an exposed person.
Copyright © 2016 Ma et al.
Figures



Similar articles
-
High beta-chemokine expression levels in lymphoid tissues of simian/human immunodeficiency virus 89.6-vaccinated rhesus macaques are associated with uncontrolled replication of simian immunodeficiency virus challenge inoculum.J Virol. 2004 Jun;78(12):6399-408. doi: 10.1128/JVI.78.12.6399-6408.2004. J Virol. 2004. PMID: 15163733 Free PMC article.
-
Immunophenotypic characterization of simian immunodeficiency virus-infected dendritic cells in cervix, vagina, and draining lymph nodes of rhesus monkeys.Lab Invest. 1998 Apr;78(4):435-51. Lab Invest. 1998. PMID: 9564888
-
SIVmac251 is inefficiently transmitted to rhesus macaques by penile inoculation with a single SIVenv variant found in ramp-up phase plasma.AIDS Res Hum Retroviruses. 2011 Dec;27(12):1259-69. doi: 10.1089/aid.2011.0090. Epub 2011 Jul 6. AIDS Res Hum Retroviruses. 2011. PMID: 21732792 Free PMC article.
-
Localization of Simian immunodeficiency virus-infected cells in the genital tract of male and female Rhesus macaques.J Reprod Immunol. 1998 Dec;41(1-2):331-9. doi: 10.1016/s0165-0378(98)00069-2. J Reprod Immunol. 1998. PMID: 10213321 Review.
-
Mucosal Vaccination for Prevention of HIV Infection and AIDS.Curr HIV Res. 2016;14(3):247-59. doi: 10.2174/1570162x14999160224103025. Curr HIV Res. 2016. PMID: 26957199 Review.
Cited by
-
The well-tempered SIV infection: Pathogenesis of SIV infection in natural hosts in the wild, with emphasis on virus transmission and early events post-infection that may contribute to protection from disease progression.Infect Genet Evol. 2016 Dec;46:308-323. doi: 10.1016/j.meegid.2016.07.006. Epub 2016 Jul 6. Infect Genet Evol. 2016. PMID: 27394696 Free PMC article.
-
Barriers of Mucosal Entry of HIV/SIV.Curr Immunol Rev. 2019;15(1):4-13. doi: 10.2174/1573395514666180604084404. Curr Immunol Rev. 2019. PMID: 31853241 Free PMC article.
-
Lymph Node Cellular and Viral Dynamics in Natural Hosts and Impact for HIV Cure Strategies.Front Immunol. 2018 Apr 19;9:780. doi: 10.3389/fimmu.2018.00780. eCollection 2018. Front Immunol. 2018. PMID: 29725327 Free PMC article. Review.
-
Paradoxical myeloid-derived suppressor cell reduction in the bone marrow of SIV chronically infected macaques.PLoS Pathog. 2017 May 12;13(5):e1006395. doi: 10.1371/journal.ppat.1006395. eCollection 2017 May. PLoS Pathog. 2017. PMID: 28498847 Free PMC article.
-
Exploring HIV Vaccine Progress in the Pre-Clinical and Clinical Setting: From History to Future Prospects.Viruses. 2024 Feb 27;16(3):368. doi: 10.3390/v16030368. Viruses. 2024. PMID: 38543734 Free PMC article. Review.
References
-
- UNAIDS. 2014. The gap report. World Health Organization and Joint United Nations Programme on HIV/AIDS, Geneva, Switzerland.
-
- Buchbinder SP, Mehrotra DV, Duerr A, Fitzgerald DW, Mogg R, Li D, Gilbert PB, Lama JR, Marmor M, Del Rio C, McElrath MJ, Casimiro DR, Gottesdiener KM, Chodakewitz JA, Corey L, Robertson MN. 2008. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet 372:1881–1893. doi:10.1016/S0140-6736(08)61591-3. - DOI - PMC - PubMed
-
- Joint United Nations Programme on HIV/AIDS. 2007. Male circumcision: global trends and determinants of prevalence, safety and acceptability. World Health Organization and Joint United Nations Programme on HIV/AIDS, Geneva, Switzerland.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

