Two Novel DNAs That Enhance Symptoms and Overcome CMD2 Resistance to Cassava Mosaic Disease
- PMID: 26865712
- PMCID: PMC4810563
- DOI: 10.1128/JVI.02834-15
Two Novel DNAs That Enhance Symptoms and Overcome CMD2 Resistance to Cassava Mosaic Disease
Abstract
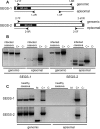
Cassava mosaic begomoviruses (CMBs) cause cassava mosaic disease (CMD) across Africa and the Indian subcontinent. Like all members of the geminivirus family, CMBs have small, circular single-stranded DNA genomes. We report here the discovery of two novel DNA sequences, designated SEGS-1 and SEGS-2 (forsequencesenhancinggeminivirussymptoms), that enhance symptoms and break resistance to CMD. The SEGS are characterized by GC-rich regions and the absence of long open reading frames. Both SEGS enhanced CMD symptoms in cassava (Manihot esculentaCrantz) when coinoculated withAfrican cassava mosaic virus(ACMV),East African cassava mosaic Cameroon virus(EACMCV), orEast African cassava mosaic virus-Uganda(EACMV-UG). SEGS-1 also overcame resistance of a cassava landrace carrying the CMD2 resistance locus when coinoculated with EACMV-UG. Episomal forms of both SEGS were detected in CMB-infected cassava but not in healthy cassava. SEGS-2 episomes were also found in virions and whiteflies. SEGS-1 has no homology to geminiviruses or their associated satellites, but the cassava genome contains a sequence that is 99% identical to full-length SEGS-1. The cassava genome also includes three sequences with 84 to 89% identity to SEGS-2 that together encompass all of SEGS-2 except for a 52-bp region, which includes the episomal junction and a 26-bp sequence related to alphasatellite replication origins. These results suggest that SEGS-1 is derived from the cassava genome and facilitates CMB infection as an integrated copy and/or an episome, while SEGS-2 was originally from the cassava genome but now is encapsidated into virions and transmitted as an episome by whiteflies.
Importance: Cassava is a major crop in the developing world, with its production in Africa being second only to maize. CMD is one of the most important diseases of cassava and a serious constraint to production across Africa. CMD2 is a major CMD resistance locus that has been deployed in many cassava cultivars through large-scale breeding programs. In recent years, severe, atypical CMD symptoms have been observed occasionally on resistant cultivars, some of which carry the CMD2 locus, in African fields. In this report, we identified and characterized two DNA sequences, SEGS-1 and SEGS-2, which produce similar symptoms when coinoculated with cassava mosaic begomoviruses onto a susceptible cultivar or a CMD2-resistant landrace. The ability of SEGS-1 to overcome CMD2 resistance and the transmission of SEGS-2 by whiteflies has major implications for the long-term durability of CMD2 resistance and underscore the need for alternative sources of resistance in cassava.
Copyright © 2016 Ndunguru et al.
Figures






Similar articles
-
A New Type of Satellite Associated with Cassava Mosaic Begomoviruses.J Virol. 2021 Oct 13;95(21):e0043221. doi: 10.1128/JVI.00432-21. Epub 2021 Aug 18. J Virol. 2021. PMID: 34406866 Free PMC article.
-
SEGS-1 episomes generated during cassava mosaic disease enhance disease severity.Front Plant Sci. 2025 Jan 10;15:1469045. doi: 10.3389/fpls.2024.1469045. eCollection 2024. Front Plant Sci. 2025. PMID: 39911651 Free PMC article.
-
Differential response of cassava genotypes to infection by cassava mosaic geminiviruses.Virus Res. 2017 Jan 2;227:69-81. doi: 10.1016/j.virusres.2016.09.022. Epub 2016 Sep 29. Virus Res. 2017. PMID: 27693919 Free PMC article.
-
Cassava Mosaic and Brown Streak Diseases: Current Perspectives and Beyond.Annu Rev Virol. 2017 Sep 29;4(1):429-452. doi: 10.1146/annurev-virology-101416-041913. Epub 2017 Jun 23. Annu Rev Virol. 2017. PMID: 28645239 Review.
-
Cassava mosaic geminiviruses in Africa.Plant Mol Biol. 2004 Nov;56(4):585-99. doi: 10.1007/s11103-004-1651-7. Plant Mol Biol. 2004. PMID: 15630622 Review.
Cited by
-
A VIGS screen identifies immunity in the Arabidopsis Pla-1 accession to viruses in two different genera of the Geminiviridae.Plant J. 2017 Dec;92(5):796-807. doi: 10.1111/tpj.13716. Epub 2017 Oct 24. Plant J. 2017. PMID: 28901681 Free PMC article.
-
DNA Satellites Impact Begomovirus Diseases in a Virus-Specific Manner.Int J Mol Sci. 2025 Jun 17;26(12):5814. doi: 10.3390/ijms26125814. Int J Mol Sci. 2025. PMID: 40565275 Free PMC article. Review.
-
A New Type of Satellite Associated with Cassava Mosaic Begomoviruses.J Virol. 2021 Oct 13;95(21):e0043221. doi: 10.1128/JVI.00432-21. Epub 2021 Aug 18. J Virol. 2021. PMID: 34406866 Free PMC article.
-
Multiple morphogenic culture systems cause loss of resistance to cassava mosaic disease.BMC Plant Biol. 2018 Jun 25;18(1):132. doi: 10.1186/s12870-018-1354-x. BMC Plant Biol. 2018. PMID: 29940871 Free PMC article.
-
Advancements and strategies of genetic improvement in cassava (Manihot esculenta Crantz): from conventional to genomic approaches.Hortic Res. 2024 Dec 2;12(3):uhae341. doi: 10.1093/hr/uhae341. eCollection 2025 Mar. Hortic Res. 2024. PMID: 40061801 Free PMC article.
References
-
- FAO. 2010. Cassava diseases in central, eastern and southern Africa (CaCESA). Food and Agriculture Organization of the United Nations, Rome, Italy.
-
- Okogbenin E, Egesi CN, Olasanmi B, Ogundapo OO, Kahya S, Hurtado P, Marin J, Akinbo O, Mba C, Gomez H, de Vicente C, Baiyeri S, Uguru M, Ewa F, Fregene M. 2012. Molecular marker analysis and validation of resistance to Cassava mosaic disease in elite cassava genotypes in Nigeria. Crop Sci 52:2576–2586. doi: 10.2135/cropsci2011.11.0586. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

