Potential To Streamline Heterologous DNA Prime and NYVAC/Protein Boost HIV Vaccine Regimens in Rhesus Macaques by Employing Improved Antigens
- PMID: 26865719
- PMCID: PMC4810551
- DOI: 10.1128/JVI.03135-15
Potential To Streamline Heterologous DNA Prime and NYVAC/Protein Boost HIV Vaccine Regimens in Rhesus Macaques by Employing Improved Antigens
Abstract
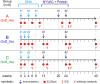
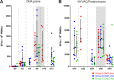
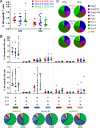
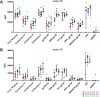
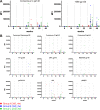
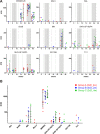
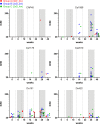
In a follow-up to the modest efficacy observed in the RV144 trial, researchers in the HIV vaccine field seek to substantiate and extend the results by evaluating other poxvirus vectors and combinations with DNA and protein vaccines. Earlier clinical trials (EuroVacc trials 01 to 03) evaluated the immunogenicity of HIV-1 clade C GagPolNef and gp120 antigens delivered via the poxviral vector NYVAC. These showed that a vaccination regimen including DNA-C priming prior to a NYVAC-C boost considerably enhanced vaccine-elicited immune responses compared to those with NYVAC-C alone. Moreover, responses were improved by using three as opposed to two DNA-C primes. In the present study, we assessed in nonhuman primates whether such vaccination regimens can be streamlined further by using fewer and accelerated immunizations and employing a novel generation of improved DNA-C and NYVAC-C vaccine candidates designed for higher expression levels and more balanced immune responses. Three different DNA-C prime/NYVAC-C+ protein boost vaccination regimens were tested in rhesus macaques. All regimens elicited vigorous and well-balanced CD8(+)and CD4(+)T cell responses that were broad and polyfunctional. Very high IgG binding titers, substantial antibody-dependent cellular cytotoxicity (ADCC), and modest antibody-dependent cell-mediated virus inhibition (ADCVI), but very low neutralization activity, were measured after the final immunizations. Overall, immune responses elicited in all three groups were very similar and of greater magnitude, breadth, and quality than those of earlier EuroVacc vaccines. In conclusion, these findings indicate that vaccination schemes can be simplified by using improved antigens and regimens. This may offer a more practical and affordable means to elicit potentially protective immune responses upon vaccination, especially in resource-constrained settings.
Importance: Within the EuroVacc clinical trials, we previously assessed the immunogenicity of HIV clade C antigens delivered in a DNA prime/NYVAC boost regimen. The trials showed that the DNA prime crucially improved the responses, and three DNA primes with a NYVAC boost appeared to be optimal. Nevertheless, T cell responses were primarily directed toward Env, and humoral responses were modest. The aim of this study was to assess improved antigens for the capacity to elicit more potent and balanced responses in rhesus macaques, even with various simpler immunization regimens. Our results showed that the novel antigens in fact elicited larger numbers of T cells with a polyfunctional profile and a good Env-GagPolNef balance, as well as high-titer and Fc-functional antibody responses. Finally, comparison of the different schedules indicates that a simpler regimen of only two DNA primes and one NYVAC boost in combination with protein may be very efficient, thus showing that the novel antigens allow for easier immunization protocols.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures


Similar articles
-
Priming with a Potent HIV-1 DNA Vaccine Frames the Quality of Immune Responses prior to a Poxvirus and Protein Boost.J Virol. 2019 Jan 17;93(3):e01529-18. doi: 10.1128/JVI.01529-18. Print 2019 Feb 1. J Virol. 2019. PMID: 30429343 Free PMC article.
-
HIV/AIDS Vaccine Candidates Based on Replication-Competent Recombinant Poxvirus NYVAC-C-KC Expressing Trimeric gp140 and Gag-Derived Virus-Like Particles or Lacking the Viral Molecule B19 That Inhibits Type I Interferon Activate Relevant HIV-1-Specific B and T Cell Immune Functions in Nonhuman Primates.J Virol. 2017 Apr 13;91(9):e02182-16. doi: 10.1128/JVI.02182-16. Print 2017 May 1. J Virol. 2017. PMID: 28179536 Free PMC article.
-
Head-to-Head Comparison of Poxvirus NYVAC and ALVAC Vectors Expressing Identical HIV-1 Clade C Immunogens in Prime-Boost Combination with Env Protein in Nonhuman Primates.J Virol. 2015 Aug;89(16):8525-39. doi: 10.1128/JVI.01265-15. Epub 2015 Jun 3. J Virol. 2015. PMID: 26041302 Free PMC article.
-
Development of a DNA-MVA/HIVA vaccine for Kenya.Vaccine. 2002 May 6;20(15):1995-8. doi: 10.1016/s0264-410x(02)00085-3. Vaccine. 2002. PMID: 11983261 Review.
-
Progress on the induction of neutralizing antibodies against HIV type 1 (HIV-1).BioDrugs. 2009;23(3):137-53. doi: 10.2165/00063030-200923030-00001. BioDrugs. 2009. PMID: 19627166 Free PMC article. Review.
Cited by
-
Priming with a Potent HIV-1 DNA Vaccine Frames the Quality of Immune Responses prior to a Poxvirus and Protein Boost.J Virol. 2019 Jan 17;93(3):e01529-18. doi: 10.1128/JVI.01529-18. Print 2019 Feb 1. J Virol. 2019. PMID: 30429343 Free PMC article.
-
Use of a Novel Enhanced DNA Vaccine Vector for Preclinical Virus Vaccine Investigation.Vaccines (Basel). 2019 Jun 13;7(2):50. doi: 10.3390/vaccines7020050. Vaccines (Basel). 2019. PMID: 31200559 Free PMC article. Review.
-
Application of area scaling analysis to identify natural killer cell and monocyte involvement in the GranToxiLux antibody dependent cell-mediated cytotoxicity assay.Cytometry A. 2018 Apr;93(4):436-447. doi: 10.1002/cyto.a.23348. Epub 2018 Mar 2. Cytometry A. 2018. PMID: 29498807 Free PMC article. Clinical Trial.
-
Computational Design of Epitope-Enriched HIV-1 Gag Antigens with Preserved Structure and Function for Induction of Broad CD8+ T Cell Responses.Sci Rep. 2018 Jul 26;8(1):11264. doi: 10.1038/s41598-018-29435-1. Sci Rep. 2018. PMID: 30050069 Free PMC article.
-
Optimal priming of poxvirus vector (NYVAC)-based HIV vaccine regimens for T cell responses requires three DNA injections. Results of the randomized multicentre EV03/ANRS VAC20 Phase I/II Trial.PLoS Pathog. 2020 Jun 26;16(6):e1008522. doi: 10.1371/journal.ppat.1008522. eCollection 2020 Jun. PLoS Pathog. 2020. PMID: 32589686 Free PMC article. Clinical Trial.
References
-
- Hansen SG, Ford JC, Lewis MS, Ventura AB, Hughes CM, Coyne-Johnson L, Whizin N, Oswald K, Shoemaker R, Swanson T, Legasse AW, Chiuchiolo MJ, Parks CL, Axthelm MK, Nelson JA, Jarvis MA, Piatak M, Lifson JD, Picker LJ. 2011. Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine. Nature 473:523–527. doi: 10.1038/nature10003. - DOI - PMC - PubMed
-
- Hansen SG, Sacha JB, Hughes CM, Ford JC, Burwitz BJ, Scholz I, Gilbride RM, Lewis MS, Gilliam AN, Ventura AB, Malouli D, Xu G, Richards R, Whizin N, Reed JS, Hammond KB, Fischer M, Turner JM, Legasse AW, Axthelm MK, Edlefsen PT, Nelson JA, Lifson JD, Früh K, Picker LJ. 2013. Cytomegalovirus vectors violate CD8+ T cell epitope recognition paradigms. Science 340:1237874. doi: 10.1126/science.1237874. - DOI - PMC - PubMed
-
- Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, Premsri N, Namwat C, de Souza M, Adams E, Benenson M, Gurunathan S, Tartaglia J, McNeil JG, Francis DP, Stablein D, Birx DL, Chunsuttiwat S, Khamboonruang C, Thongcharoen P, Robb ML, Michael NL, Kunasol P, Kim JH. 2009. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med 361:2209–2220. doi: 10.1056/NEJMoa0908492. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

