Hepatitis C Virus-Induced Degradation of Cell Death-Inducing DFFA-Like Effector B Leads to Hepatic Lipid Dysregulation
- PMID: 26865724
- PMCID: PMC4810547
- DOI: 10.1128/JVI.02891-15
Hepatitis C Virus-Induced Degradation of Cell Death-Inducing DFFA-Like Effector B Leads to Hepatic Lipid Dysregulation
Abstract
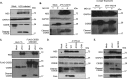
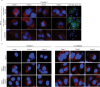
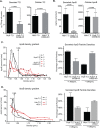
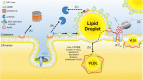
Individuals chronically infected with hepatitis C virus (HCV) commonly exhibit hepatic intracellular lipid accumulation, termed steatosis. HCV infection perturbs host lipid metabolism through both cellular and virus-induced mechanisms, with the viral core protein playing an important role in steatosis development. We have recently identified a liver protein, the cell death-inducing DFFA-like effector B (CIDEB), as an HCV entry host dependence factor that is downregulated by HCV infection in a cell culture model. In this study, we investigated the biological significance and molecular mechanism of this downregulation. HCV infection in a mouse model downregulated CIDEB in the liver tissue, and knockout of the CIDEB gene in a hepatoma cell line results in multiple aspects of lipid dysregulation that can contribute to hepatic steatosis, including reduced triglyceride secretion, lower lipidation of very-low-density lipoproteins, and increased lipid droplet (LD) stability. The potential link between CIDEB downregulation and steatosis is further supported by the requirement of the HCV core and its LD localization for CIDEB downregulation, which utilize a proteolytic cleavage event that is independent of the cellular proteasomal degradation of CIDEB.
Importance: Our data demonstrate that HCV infection of human hepatocytesin vitroandin vivoresults in CIDEB downregulation via a proteolytic cleavage event. Reduction of CIDEB protein levels by HCV or gene editing, in turn, leads to multiple aspects of lipid dysregulation, including LD stabilization. Consequently, CIDEB downregulation may contribute to HCV-induced hepatic steatosis.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures




Similar articles
-
Cell-death-inducing DFFA-like Effector B Contributes to the Assembly of Hepatitis C Virus (HCV) Particles and Interacts with HCV NS5A.Sci Rep. 2016 Jun 10;6:27778. doi: 10.1038/srep27778. Sci Rep. 2016. PMID: 27282740 Free PMC article.
-
Cell death-inducing DFFA-like effector b is required for hepatitis C virus entry into hepatocytes.J Virol. 2014 Aug;88(15):8433-44. doi: 10.1128/JVI.00081-14. Epub 2014 May 14. J Virol. 2014. PMID: 24829338 Free PMC article.
-
Investigating the antiviral role of cell death-inducing DFF45-like effector B in HCV replication.FEBS J. 2014 Aug;281(16):3751-65. doi: 10.1111/febs.12901. Epub 2014 Jul 23. FEBS J. 2014. PMID: 24980280
-
Pathogenesis and significance of hepatitis C virus steatosis: an update on survival strategy of a successful pathogen.World J Gastroenterol. 2014 Jun 21;20(23):7089-103. doi: 10.3748/wjg.v20.i23.7089. World J Gastroenterol. 2014. PMID: 24966582 Free PMC article. Review.
-
Hepatitis C virus hijacks host lipid metabolism.Trends Endocrinol Metab. 2010 Jan;21(1):33-40. doi: 10.1016/j.tem.2009.07.005. Epub 2009 Oct 23. Trends Endocrinol Metab. 2010. PMID: 19854061 Free PMC article. Review.
Cited by
-
HCV Interplay with Lipoproteins: Inside or Outside the Cells?Viruses. 2020 Apr 12;12(4):434. doi: 10.3390/v12040434. Viruses. 2020. PMID: 32290553 Free PMC article. Review.
-
Cell-death-inducing DFFA-like Effector B Contributes to the Assembly of Hepatitis C Virus (HCV) Particles and Interacts with HCV NS5A.Sci Rep. 2016 Jun 10;6:27778. doi: 10.1038/srep27778. Sci Rep. 2016. PMID: 27282740 Free PMC article.
References
-
- Pawlotsky JM. 2013. Treatment of chronic hepatitis C: current and future. Curr Top Microbiol Immunol 369:321–342. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

