Porcine Sapelovirus Uses α2,3-Linked Sialic Acid on GD1a Ganglioside as a Receptor
- PMID: 26865725
- PMCID: PMC4810533
- DOI: 10.1128/JVI.02449-15
Porcine Sapelovirus Uses α2,3-Linked Sialic Acid on GD1a Ganglioside as a Receptor
Abstract
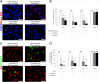
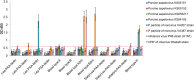
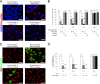
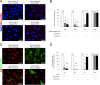
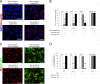
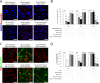
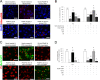
The receptor(s) for porcine sapelovirus (PSV), which causes diarrhea, pneumonia, polioencephalomyelitis, and reproductive disorders in pigs, remains largely unknown. Given the precedent for other picornaviruses which use terminal sialic acids (SAs) as receptors, we examined the role of SAs in PSV binding and infection. Using a variety of approaches, including treating cells with a carbohydrate-destroying chemical (NaIO4), mono- or oligosaccharides (N-acetylneuraminic acid, galactose, and 6'-sialyllactose), linkage-specific sialidases (neuraminidase and sialidase S), lectins (Maakia amurensislectin andSambucus nigralectin), proteases (trypsin and chymotrypsin), and glucosylceramide synthase inhibitors (dl-threo-1-phenyl-2-decanoylamino-3-morpholino-1-propanol and phospholipase C), we demonstrated that PSV could recognize α2,3-linked SA on glycolipids as a receptor. On the other hand, PSVs had no binding affinity for synthetic histo-blood group antigens (HBGAs), suggesting that PSVs could not use HBGAs as receptors. Depletion of cell surface glycolipids followed by reconstitution studies indicated that GD1a ganglioside, but not other gangliosides, could restore PSV binding and infection, further confirming α2,3-linked SA on GD1a as a PSV receptor. Our results could provide significant information on the understanding of the life cycle of sapelovirus and other picornaviruses. For the broader community in the area of pathogens and pathogenesis, these findings and insights could contribute to the development of affordable, useful, and efficient drugs for anti-sapelovirus therapy.
Importance: The porcine sapelovirus (PSV) is known to cause enteritis, pneumonia, polioencephalomyelitis, and reproductive disorders in pigs. However, the receptor(s) that the PSV utilizes to enter host cells remains largely unknown. Using a variety of approaches, we showed that α2,3-linked terminal sialic acid (SA) on the cell surface GD1a ganglioside could be used for PSV binding and infection as a receptor. On the other hand, histo-blood group antigens also present in the cell surface carbohydrates could not be utilized as PSV receptors for binding and infection. These findings should contribute to the understanding of the sapelovirus life cycle and to the development of affordable, useful and efficient drugs for anti-sapelovirus therapy.
Copyright © 2016 Kim et al.
Figures
References
-
- Kim DS, Hosmillo M, Alfajaro MM, Kim JY, Park JG, Son KY, Ryu EH, Sorgeloos F, Kwon HJ, Park SJ, Lee WS, Cho D, Kwon J, Choi JS, Kang MI, Goodfellow I, Cho KO. 2014. Both α2,3- and 2,6-linked sialic acids on O-linked glycoproteins act as functional receptors for porcine sapovirus. PLoS Pathog 10:e1004172. doi:10.1371/journal.ppat.1004172. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

