In vitro methods to study bubble-cell interactions: Fundamentals and therapeutic applications
- PMID: 26865903
- PMCID: PMC4733084
- DOI: 10.1063/1.4940429
In vitro methods to study bubble-cell interactions: Fundamentals and therapeutic applications
Abstract
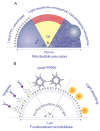
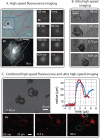
Besides their use as contrast agents for ultrasound imaging, microbubbles are increasingly studied for a wide range of therapeutic applications. In particular, their ability to enhance the uptake of drugs through the permeabilization of tissues and cell membranes shows great promise. In order to fully understand the numerous paths by which bubbles can interact with cells and the even larger number of possible biological responses from the cells, thorough and extensive work is necessary. In this review, we consider the range of experimental techniques implemented in in vitro studies with the aim of elucidating these microbubble-cell interactions. First of all, the variety of cell types and cell models available are discussed, emphasizing the need for more and more complex models replicating in vivo conditions together with experimental challenges associated with this increased complexity. Second, the different types of stabilized microbubbles and more recently developed droplets and particles are presented, followed by their acoustic or optical excitation methods. Finally, the techniques exploited to study the microbubble-cell interactions are reviewed. These techniques operate over a wide range of timescales, or even off-line, revealing particular aspects or subsequent effects of these interactions. Therefore, knowledge obtained from several techniques must be combined to elucidate the underlying processes.
Figures


Similar articles
-
The Role of Ultrasound-Driven Microbubble Dynamics in Drug Delivery: From Microbubble Fundamentals to Clinical Translation.Langmuir. 2019 Aug 6;35(31):10173-10191. doi: 10.1021/acs.langmuir.8b03779. Epub 2019 Feb 4. Langmuir. 2019. PMID: 30653325 Review.
-
WE-C-218-01: Ultrasound Contrast Agents.Med Phys. 2012 Jun;39(6Part27):3953. doi: 10.1118/1.4736133. Med Phys. 2012. PMID: 28520019
-
Droplets, Bubbles and Ultrasound Interactions.Adv Exp Med Biol. 2016;880:157-74. doi: 10.1007/978-3-319-22536-4_9. Adv Exp Med Biol. 2016. PMID: 26486337 Review.
-
Interaction of an ultrasound-activated contrast microbubble with a wall at arbitrary separation distances.Phys Med Biol. 2015 Oct 21;60(20):7909-25. doi: 10.1088/0031-9155/60/20/7909. Epub 2015 Sep 25. Phys Med Biol. 2015. PMID: 26407104
-
Ultrasound contrast agents: basic principles.Eur J Radiol. 1998 May;27 Suppl 2:S157-60. doi: 10.1016/s0720-048x(98)00057-6. Eur J Radiol. 1998. PMID: 9652516 Review.
Cited by
-
Pharmacokinetics and pharmacodynamics of perfluoropropane after intra-venous bolus injection of perflutren lipid microsphere injection (DEFINITY®) in healthy Chinese volunteers.BMC Pharmacol Toxicol. 2024 Jan 2;25(1):6. doi: 10.1186/s40360-023-00729-z. BMC Pharmacol Toxicol. 2024. PMID: 38167238 Free PMC article.
-
Layered acoustofluidic resonators for the simultaneous optical and acoustic characterisation of cavitation dynamics, microstreaming, and biological effects.Biomicrofluidics. 2018 May 30;12(3):034109. doi: 10.1063/1.5023729. eCollection 2018 May. Biomicrofluidics. 2018. PMID: 29887932 Free PMC article.
-
Sonoporation of Cells by a Parallel Stable Cavitation Microbubble Array.Adv Sci (Weinh). 2019 Jun 17;6(17):1900557. doi: 10.1002/advs.201900557. eCollection 2019 Sep 4. Adv Sci (Weinh). 2019. PMID: 31508275 Free PMC article.
-
Clinical study of ultrasound and microbubbles for enhancing chemotherapeutic sensitivity of malignant tumors in digestive system.Chin J Cancer Res. 2018 Oct;30(5):553-563. doi: 10.21147/j.issn.1000-9604.2018.05.09. Chin J Cancer Res. 2018. PMID: 30510367 Free PMC article.
-
Sonoporation Using Nanoparticle-Loaded Microbubbles Increases Cellular Uptake of Nanoparticles Compared to Co-Incubation of Nanoparticles and Microbubbles.Pharmaceutics. 2021 Apr 30;13(5):640. doi: 10.3390/pharmaceutics13050640. Pharmaceutics. 2021. PMID: 33946327 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
