Signaling Pathways in Osteoclast Differentiation
- PMID: 26865996
- PMCID: PMC4742606
- DOI: 10.4068/cmj.2016.52.1.12
Signaling Pathways in Osteoclast Differentiation
Abstract
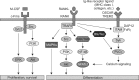
Osteoclasts are multinucleated cells of hematopoietic origin that are responsible for the degradation of old bone matrix. Osteoclast differentiation and activity are controlled by two essential cytokines, macrophage colony-stimulating factor (M-CSF) and the receptor activator of nuclear factor-κB ligand (RANKL). M-CSF and RANKL bind to their respective receptors c-Fms and RANK to stimulate osteoclast differentiation through regulation of delicate signaling systems. Here, we summarize the critical or essential signaling pathways for osteoclast differentiation including M-CSF-c-Fms signaling, RANKL-RANK signaling, and costimulatory signaling for RANK.
Keywords: Bone and bones; Macrophage colony-stimulating factor; Osteoclasts; RANK ligand; Signal transduction.
Conflict of interest statement
Figures
References
-
- Takayanagi H. Osteoimmunology: shared mechanisms and crosstalk between the immune and bone systems. Nat Rev Immunol. 2007;7:292–304. - PubMed
-
- Seeman E, Delmas PD. Bone quality--the material and structural basis of bone strength and fragility. N Engl J Med. 2006;354:2250–2261. - PubMed
-
- Teitelbaum SL, Ross FP. Genetic regulation of osteoclast development and function. Nat Rev Genet. 2003;4:638–649. - PubMed
-
- Sherr CJ. Colony-stimulating factor-1 receptor. Blood. 1990;75:1–12. - PubMed
-
- Suda T, Takahashi N, Udagawa N, Jimi E, Gillespie MT, Martin TJ. Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocr Rev. 1999;20:345–357. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

