Desensitization and Incomplete Recovery of Hepatic Target Genes After Chronic Thyroid Hormone Treatment and Withdrawal in Male Adult Mice
- PMID: 26866609
- PMCID: PMC4816733
- DOI: 10.1210/en.2015-1848
Desensitization and Incomplete Recovery of Hepatic Target Genes After Chronic Thyroid Hormone Treatment and Withdrawal in Male Adult Mice
Abstract
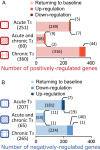
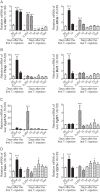
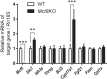
Clinical symptoms may vary and not necessarily reflect serum thyroid hormone (TH) levels during acute and chronic hyperthyroidism as well as recovery from hyperthyroidism. We thus examined changes in hepatic gene expression and serum TH/TSH levels in adult male mice treated either with a single T3 (20 μg per 100 g body weight) injection (acute T3) or daily injections for 14 days (chronic T3) followed by 10 days of withdrawal. Gene expression arrays from livers harvested at these time points showed that among positively-regulated target genes, 320 were stimulated acutely and 429 chronically by T3. Surprisingly, only 69 of 680 genes (10.1%) were induced during both periods, suggesting desensitization of the majority of acutely stimulated target genes. About 90% of positively regulated target genes returned to baseline expression levels after 10 days of withdrawal; however, 67 of 680 (9.9%) did not return to baseline despite normalization of serum TH/TSH levels. Similar findings also were observed for negatively regulated target genes. Chromatin immunoprecipitation analysis of representative positively regulated target genes suggested that acetylation of H3K9/K14 was associated with acute stimulation, whereas trimethylation of H3K4 was associated with chronic stimulation. In an in vivo model of chronic intrahepatic hyperthyroidism since birth, adult male monocarboxylate transporter-8 knockout mice also demonstrated desensitization of most acutely stimulated target genes that were examined. In summary, we have identified transcriptional desensitization and incomplete recovery of gene expression during chronic hyperthyroidism and recovery. Our findings may be a potential reason for discordance between clinical symptoms and serum TH levels observed in these conditions.
Figures

Comment in
-
Differential Hepatic Gene Expression Profile and Histone Modification After Acute or Chronic Hyperthyroidism or During Remission.Endocrinology. 2016 Apr;157(4):1326-7. doi: 10.1210/en.2016-1110. Endocrinology. 2016. PMID: 27035771 No abstract available.
References
-
- Fahrenfort JJ, Wilterdink AM, van der Veen EA. Long-term residual complaints and psychosocial sequelae after remission of hyperthyroidism. Psychoneuroendocrinology. 2000;25:201–211. - PubMed
-
- Piatnek-Leunissen DA, Leunissen RL. Liver mitochondrial function in acute vs. chronic hyperthyroidism. Endocrinology. 1969;84:456–461. - PubMed
-
- Varas SM, Oliveros LB, Gimenez MS. Lipids in rat liver submitted to acute and chronic hyperthyroidism. Horm Metab Res. 1999;31:514–518. - PubMed
-
- Sarne DH, Refetoff S, Rosenfield RL, Farriaux JP. Sex hormone-binding globulin in the diagnosis of peripheral tissue resistance to thyroid hormone: the value of changes after short term triiodothyronine administration. J Clin Endocrinol Metab. 1988;66:740–746. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

