C6: A Monoclonal Antibody Specific for a Fibronectin Epitope Situated at the Interface between the Oncofoetal Extra-Domain B and the Repeat III8
- PMID: 26867013
- PMCID: PMC4750999
- DOI: 10.1371/journal.pone.0148103
C6: A Monoclonal Antibody Specific for a Fibronectin Epitope Situated at the Interface between the Oncofoetal Extra-Domain B and the Repeat III8
Abstract
Background: Fibronectin (FN) is a large multidomain molecule that is involved in many cellular processes. Different FN isoforms arise from alternative splicing of the pre-mRNA including, most notably, the FN isoform that contains the "extra-domain-B" (ED-B). The FN isoform containing ED-B (known as B-FN) is undetectable in healthy adult tissues but is present in large amounts in neoplastic and foetal tissues as well as on the blood vessels during angiogenesis. Thus, antibodies specific for B-FN can be useful for detecting and targeting neoplastic tissues in vivo. We previously characterised C6, a new monoclonal antibody specific for human B-FN and we suggested that it reacts with the B-C loop of the type III repeat 8 which is masked in FN isoforms lacking ED-B and that the insertion of ED-B in FN molecules unmasked it. Here we have now consolidated and refined the characterization of this B-FN specific antibody demonstrating that the epitope recognized by C6 also includes loop E-F of ED-B.
Methodology: We built the three dimensional model of the variable regions of the mAb C6 and of the FN fragment EDB-III8 and performed protein:protein docking simulation using the web server ClusPro2.0. To confirm the data obtained by protein:protein docking we generated mutant fragments of the recombinant FN fragment EDB-III8 and tested their reactivity with C6.
Conclusion: The monoclonal antibody C6 reacts with an epitope formed by the B-C loop of domain III8 and the E-F loop of ED-B. Both loops are required for an immunological reaction, thus this monoclonal is strictly specific for B-FN but the part of the epitope on III8 confers the human specificity.
Conflict of interest statement
Figures
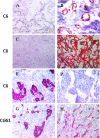



Similar articles
-
Alternative splicing of the angiogenesis associated extra-domain B of fibronectin regulates the accessibility of the B-C loop of the type III repeat 8.PLoS One. 2010 Feb 10;5(2):e9145. doi: 10.1371/journal.pone.0009145. PLoS One. 2010. PMID: 20161770 Free PMC article.
-
A novel human fibronectin cryptic sequence unmasked by the insertion of the angiogenesis-associated extra type III domain B.Int J Cancer. 2009 Aug 15;125(4):751-8. doi: 10.1002/ijc.24473. Int J Cancer. 2009. PMID: 19479996
-
NMR structure of the human oncofoetal fibronectin ED-B domain, a specific marker for angiogenesis.Structure. 1999 Apr 15;7(4):381-90. doi: 10.1016/s0969-2126(99)80051-3. Structure. 1999. PMID: 10196121
-
Diagnostic and therapeutic applications of recombinant antibodies: targeting the extra-domain B of fibronectin, a marker of tumor angiogenesis.Curr Pharm Des. 2004;10(13):1537-49. doi: 10.2174/1381612043384808. Curr Pharm Des. 2004. PMID: 15134574 Review.
-
99mTc-Anti-ED-B fibronectin single-chain antibody fragment L19-His.2007 Feb 12 [updated 2008 Jan 16]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2007 Feb 12 [updated 2008 Jan 16]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 20641593 Free Books & Documents. Review.
Cited by
-
EDB-FN targeted probes for the surgical navigation, radionuclide imaging, and therapy of thyroid cancer.Eur J Nucl Med Mol Imaging. 2023 Jun;50(7):2100-2113. doi: 10.1007/s00259-023-06147-x. Epub 2023 Feb 18. Eur J Nucl Med Mol Imaging. 2023. PMID: 36807768
-
Serum Liberation of Fetal Fibronectin Variants in Patients with Pulmonary Hypertension: ED-A+ Fn as Promising Novel Biomarker of Pulmonary Vascular and Right Ventricular Myocardial Remodeling.J Clin Med. 2021 Jun 9;10(12):2559. doi: 10.3390/jcm10122559. J Clin Med. 2021. PMID: 34207881 Free PMC article.
References
-
- Yamada KM, Clark RAF. Provisional Matrix In: Clark RAF, ed. The Molecular and Cellular Biology of Wound Repair. New York: Plenum Press; 1996;51–93.
-
- Pankov R, Yamada KM. Fibronectin at a glance. J Cell Sci. 2002;115(Pt 20):3861–3. - PubMed
-
- Kosmehl H, Berndt A, Katenkamp D. Molecular variants of fibronectin and laminin: structure, physiological occurrence and histopathological aspects. Virchows Arch. 1996;429(6):311–22. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

