IL-17a and IL-22 Induce Expression of Antimicrobials in Gastrointestinal Epithelial Cells and May Contribute to Epithelial Cell Defense against Helicobacter pylori
- PMID: 26867135
- PMCID: PMC4750979
- DOI: 10.1371/journal.pone.0148514
IL-17a and IL-22 Induce Expression of Antimicrobials in Gastrointestinal Epithelial Cells and May Contribute to Epithelial Cell Defense against Helicobacter pylori
Abstract
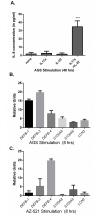
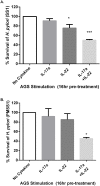
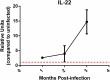
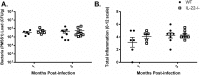
Helicobacter pylori colonization of the human stomach can lead to adverse clinical outcomes including gastritis, peptic ulcers, or gastric cancer. Current data suggest that in addition to bacterial virulence factors, the magnitude and types of immune responses influence the outcome of colonization. Specifically, CD4+ T cell responses impact the pathology elicited in response to H. pylori. Because gastritis is believed to be the initiating host response to more detrimental pathological outcomes, there has been a significant interest in pro-inflammatory T cell cytokines, including the cytokines produced by T helper 17 cells. Th17 cells produce IL-17A, IL-17F, IL-21 and IL-22. While these cytokines have been linked to inflammation, IL-17A and IL-22 are also associated with anti-microbial responses and control of bacterial colonization. The goal of this research was to determine the role of IL-22 in activation of antimicrobial responses in models of H. pylori infection using human gastric epithelial cell lines and the mouse model of H. pylori infection. Our data indicate that IL-17A and IL-22 work synergistically to induce antimicrobials and chemokines such as IL-8, components of calprotectin (CP), lipocalin (LCN) and some β-defensins in both human and primary mouse gastric epithelial cells (GEC) and gastroids. Moreover, IL-22 and IL-17A-activated GECs were capable of inhibiting growth of H. pylori in vitro. While antimicrobials were activated by IL-17A and IL-22 in vitro, using a mouse model of H. pylori infection, the data herein indicate that IL-22 deficiency alone does not render mice more susceptible to infection, change their antimicrobial gene transcription, or significantly change their inflammatory response.
Conflict of interest statement
Figures
References
-
- Bauer B, Meyer TF. The Human Gastric Pathogen Helicobacter pylori and Its Association with Gastric Cancer and Ulcer Disease. Ulcer. 2011;Article ID 340157:23. PubMed Central PMCID: PMCNone.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

