The Interaction between Respiratory Pathogens and Mucus
- PMID: 26867175
- PMCID: PMC4752725
- DOI: 10.1016/j.chom.2016.01.001
The Interaction between Respiratory Pathogens and Mucus
Abstract
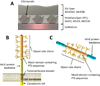
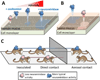
The interaction between respiratory pathogens and their hosts is complex and incompletely understood. This is particularly true when pathogens encounter the mucus layer covering the respiratory tract. The mucus layer provides an essential first host barrier to inhaled pathogens that can prevent pathogen invasion and subsequent infection. Respiratory mucus has numerous functions and interactions, both with the host and with pathogens. This review summarizes the current understanding of respiratory mucus and its interactions with the respiratory pathogens Pseudomonas aeruginosa, respiratory syncytial virus and influenza viruses, with particular focus on influenza virus transmissibility and host-range specificity. Based on current findings we propose that respiratory mucus represents an understudied host-restriction factor for influenza virus.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

