Distribution and Abundance of Glucocorticoid and Mineralocorticoid Receptors throughout the Brain of the Great Tit (Parus major)
- PMID: 26867218
- PMCID: PMC4750984
- DOI: 10.1371/journal.pone.0148516
Distribution and Abundance of Glucocorticoid and Mineralocorticoid Receptors throughout the Brain of the Great Tit (Parus major)
Abstract
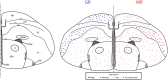
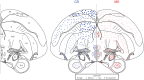
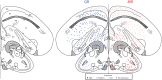
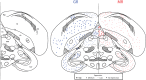
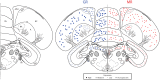
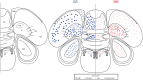
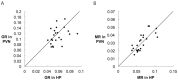
The glucocorticoid stress response, regulated by the hypothalamic-pituitary-adrenal (HPA) axis, enables individuals to cope with stressors through transcriptional effects in cells expressing the appropriate receptors. The two receptors that bind glucocorticoids-the mineralocorticoid receptor (MR) and glucocorticoid receptor (GR)-are present in a variety of vertebrate tissues, but their expression in the brain is especially important. Neural receptor patterns have the potential to integrate multiple behavioral and physiological traits simultaneously, including self-regulation of glucocorticoid secretion through negative feedback processes. In the present work, we quantified the expression of GR and MR mRNA throughout the brain of a female great tit (Parus major), creating a distribution map encompassing 48 regions. This map, the first of its kind for P. major, demonstrated a widespread but not ubiquitous distribution of both receptor types. In the paraventricular nucleus of the hypothalamus (PVN) and the hippocampus (HP)-the two brain regions that we sampled from a total of 25 birds, we found high GR mRNA expression in the former and, unexpectedly, low MR mRNA in the latter. We examined the covariation of MR and GR levels in these two regions and found a strong, positive relationship between MR in the PVN and MR in the HP and a similar trend for GR across these two regions. This correlation supports the idea that hormone pleiotropy may constrain an individual's behavioral and physiological phenotype. In the female song system, we found moderate GR in hyperstriatum ventrale, pars caudalis (HVC), and moderate MR in robust nucleus of the arcopallium (RA). Understanding intra- and interspecific patterns of glucocorticoid receptor expression can inform us about the behavioral processes (e.g. song learning) that may be sensitive to stress and stimulate future hypotheses concerning the relationships between receptor expression, circulating hormone concentrations and performance traits under selection, including behavior.
Conflict of interest statement
Figures


Similar articles
-
Changes in the expression of corticotrophin-releasing hormone, mineralocorticoid receptor and glucocorticoid receptor mRNAs in the hypothalamic paraventricular nucleus induced by fornix transection and adrenalectomy.J Neuroendocrinol. 2007 Apr;19(4):229-38. doi: 10.1111/j.1365-2826.2006.01519.x. J Neuroendocrinol. 2007. PMID: 17244200
-
Ontogeny of hypothalamic glucocorticoid receptor-mediated inhibition of the hypothalamic-pituitary-adrenal axis in mice.Stress. 2015;18(4):400-7. doi: 10.3109/10253890.2015.1046832. Epub 2015 Jun 11. Stress. 2015. PMID: 26068518 Free PMC article.
-
Lack of decrease in hypothalamic and hippocampal glucocorticoid receptor mRNA during starvation.Neuroendocrinology. 2001 Aug;74(2):120-8. doi: 10.1159/000054677. Neuroendocrinology. 2001. PMID: 11474219
-
Dissection of glucocorticoid receptor-mediated inhibition of the hypothalamic-pituitary-adrenal axis by gene targeting in mice.Front Neuroendocrinol. 2015 Jan;36:150-64. doi: 10.1016/j.yfrne.2014.09.002. Epub 2014 Sep 27. Front Neuroendocrinol. 2015. PMID: 25256348 Free PMC article. Review.
-
Aging is associated in the 344/N Fischer rat with decreased stress responsivity of central and peripheral catecholaminergic systems and impairment of the hypothalamic-pituitary-adrenal axis.Ann N Y Acad Sci. 1995 Dec 29;771:491-511. doi: 10.1111/j.1749-6632.1995.tb44705.x. Ann N Y Acad Sci. 1995. PMID: 8597425 Review.
Cited by
-
Social information changes stress hormone receptor expression in the songbird brain.Horm Behav. 2018 Jan;97:31-38. doi: 10.1016/j.yhbeh.2017.10.002. Epub 2017 Nov 7. Horm Behav. 2018. PMID: 29030109 Free PMC article.
-
Reproduction-Associated Hormones and Adult Hippocampal Neurogenesis.Neural Plast. 2021 Sep 10;2021:3651735. doi: 10.1155/2021/3651735. eCollection 2021. Neural Plast. 2021. PMID: 34539776 Free PMC article. Review.
-
Risk-averse personalities have a systemically potentiated neuroendocrine stress axis: A multilevel experiment in Parus major.Horm Behav. 2017 Jul;93:99-108. doi: 10.1016/j.yhbeh.2017.05.011. Epub 2017 May 26. Horm Behav. 2017. PMID: 28545898 Free PMC article.
-
[Effect of glucocorticoid receptor function on the behavior of rats with attention deficit hyperactivity disorder].Zhongguo Dang Dai Er Ke Za Zhi. 2018 Oct;20(10):848-853. doi: 10.7499/j.issn.1008-8830.2018.10.013. Zhongguo Dang Dai Er Ke Za Zhi. 2018. PMID: 30369362 Free PMC article. Chinese.
-
11β-HSD Types 1 and 2 in the Songbird Brain.Front Endocrinol (Lausanne). 2018 Mar 12;9:86. doi: 10.3389/fendo.2018.00086. eCollection 2018. Front Endocrinol (Lausanne). 2018. PMID: 29593652 Free PMC article.
References
-
- Nelson RJ. An introduction to behavioral endocrinology. 4th ed Sunderland: Sinauer Associates; 2005.
-
- De Kloet ER. Brain Corticosteroid Receptor Balance and Homeostatic Control. Front Neuroendocr. 1991;12: 95–164.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

