Age-Dependent Changes in Geometry, Tissue Composition and Mechanical Properties of Fetal to Adult Cryopreserved Human Heart Valves
- PMID: 26867221
- PMCID: PMC4750936
- DOI: 10.1371/journal.pone.0149020
Age-Dependent Changes in Geometry, Tissue Composition and Mechanical Properties of Fetal to Adult Cryopreserved Human Heart Valves
Abstract
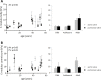
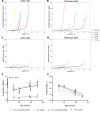
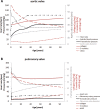
There is limited information about age-specific structural and functional properties of human heart valves, while this information is key to the development and evaluation of living valve replacements for pediatric and adolescent patients. Here, we present an extended data set of structure-function properties of cryopreserved human pulmonary and aortic heart valves, providing age-specific information for living valve replacements. Tissue composition, morphology, mechanical properties, and maturation of leaflets from 16 pairs of structurally unaffected aortic and pulmonary valves of human donors (fetal-53 years) were analyzed. Interestingly, no major differences were observed between the aortic and pulmonary valves. Valve annulus and leaflet dimensions increase throughout life. The typical three-layered leaflet structure is present before birth, but becomes more distinct with age. After birth, cell numbers decrease rapidly, while remaining cells obtain a quiescent phenotype and reside in the ventricularis and spongiosa. With age and maturation-but more pronounced in aortic valves-the matrix shows an increasing amount of collagen and collagen cross-links and a reduction in glycosaminoglycans. These matrix changes correlate with increasing leaflet stiffness with age. Our data provide a new and comprehensive overview of the changes of structure-function properties of fetal to adult human semilunar heart valves that can be used to evaluate and optimize future therapies, such as tissue engineering of heart valves. Changing hemodynamic conditions with age can explain initial changes in matrix composition and consequent mechanical properties, but cannot explain the ongoing changes in valve dimensions and matrix composition at older age.
Conflict of interest statement
Figures
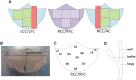

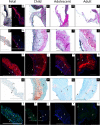
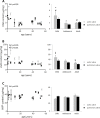
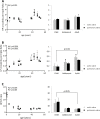
References
-
- Ross D, Jackson M, Davies J. The pulmonary autograft—a permanent aortic valve. Eur J cardio-thoracic Surg. 1992;6: 113–116. - PubMed
-
- Stephens EH, Grande-Allen KJ. Age-related changes in collagen synthesis and turnover in porcine heart valves. J Heart Valve Dis. 2007;16: 672–682. - PubMed
-
- Stephens EH, Chu C-K, Grande-Allen KJ. Valve proteoglycan content and glycosaminoglycan fine structure are unique to microstructure, mechanical load and age: Relevance to an age-specific tissue-engineered heart valve. Acta Biomater. 2008;4: 1148–1160. 10.1016/j.actbio.2008.03.014 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

