Prioritization of HCV treatment in the direct-acting antiviral era: An economic evaluation
- PMID: 26867489
- PMCID: PMC4914770
- DOI: 10.1016/j.jhep.2016.02.007
Prioritization of HCV treatment in the direct-acting antiviral era: An economic evaluation
Abstract
Background & aims: We determined the optimal HCV treatment prioritization strategy for interferon-free (IFN-free) HCV direct-acting antivirals (DAAs) by disease stage and risk status incorporating treatment of people who inject drugs (PWID).
Methods: A dynamic HCV transmission and progression model compared the cost-effectiveness of treating patients early vs. delaying until cirrhosis for patients with mild or moderate fibrosis, where PWID chronic HCV prevalence was 20, 40 or 60%. Treatment duration was 12weeks at £3300/wk, to achieve a 95% sustained viral response and was varied by genotype/stage in alternative scenarios. We estimated long-term health costs (in £UK=€1.3=$1.5) and outcomes as quality adjusted life-years (QALYs) gained using a £20,000 willingness to pay per QALY threshold. We ranked strategies with net monetary benefit (NMB); negative NMB implies delay treatment.
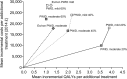
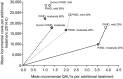
Results: The most cost-effective group to treat were PWID with moderate fibrosis (mean NMB per early treatment £60,640/£23,968 at 20/40% chronic prevalence, respectively), followed by PWID with mild fibrosis (NMB £59,258 and £19,421, respectively) then ex-PWID/non-PWID with moderate fibrosis (NMB £9,404). Treatment of ex-PWID/non-PWID with mild fibrosis could be delayed (NMB -£3,650). In populations with 60% chronic HCV among PWID it was only cost-effective to prioritize DAAs to ex-PWID/non-PWID with moderate fibrosis. For every one PWID in the 20% chronic HCV setting, 2 new HCV infections were averted. One extra HCV-related death was averted per 13 people with moderate disease treated. Rankings were unchanged with reduced drug costs or varied sustained virological response/duration by genotype/fibrosis stage.
Conclusions: Treating PWID with moderate or mild HCV with IFN-free DAAs is cost-effective compared to delay until cirrhosis, except when chronic HCV prevalence and reinfection risk is very high.
Keywords: Hepatitis C; People who inject drugs; Prevention; Treatment.
Copyright © 2016 The Authors. Published by Elsevier B.V. All rights reserved.
Figures
Comment in
-
Doing the math on hepatitis C virus treatment.J Hepatol. 2016 Jul;65(1):5-6. doi: 10.1016/j.jhep.2016.03.015. Epub 2016 Apr 2. J Hepatol. 2016. PMID: 27050632 Free PMC article. No abstract available.
Similar articles
-
Is increased hepatitis C virus case-finding combined with current or 8-week to 12-week direct-acting antiviral therapy cost-effective in UK prisons? A prevention benefit analysis.Hepatology. 2016 Jun;63(6):1796-808. doi: 10.1002/hep.28497. Epub 2016 Mar 22. Hepatology. 2016. PMID: 26864802 Free PMC article.
-
Cost-effectiveness of hepatitis C virus (HCV) elimination strategies among people who inject drugs (PWID) in Tijuana, Mexico.Addiction. 2021 Oct;116(10):2734-2745. doi: 10.1111/add.15456. Epub 2021 Mar 22. Addiction. 2021. PMID: 33620750 Free PMC article.
-
Cost-effectiveness of treating chronic hepatitis C virus with direct-acting antivirals in people who inject drugs in Australia.J Gastroenterol Hepatol. 2016 Apr;31(4):872-82. doi: 10.1111/jgh.13223. J Gastroenterol Hepatol. 2016. PMID: 26514998
-
Hepatitis C virus treatment as prevention in people who inject drugs: testing the evidence.Curr Opin Infect Dis. 2015 Dec;28(6):576-82. doi: 10.1097/QCO.0000000000000216. Curr Opin Infect Dis. 2015. PMID: 26524330 Free PMC article. Review.
-
Eligibility of persons who inject drugs for treatment of hepatitis C virus infection.World J Gastroenterol. 2014 Sep 28;20(36):12722-33. doi: 10.3748/wjg.v20.i36.12722. World J Gastroenterol. 2014. PMID: 25278674 Free PMC article. Review.
Cited by
-
Cost-effectiveness analysis of an outreach model of Hepatitis C Virus (HCV) assessment to facilitate HCV treatment in primary care.PLoS One. 2020 Jun 17;15(6):e0234577. doi: 10.1371/journal.pone.0234577. eCollection 2020. PLoS One. 2020. PMID: 32555696 Free PMC article.
-
Impact of universal access to hepatitis C therapy on HIV-infected patients: implementation of the Spanish national hepatitis C strategy.Eur J Clin Microbiol Infect Dis. 2017 Mar;36(3):487-494. doi: 10.1007/s10096-016-2822-6. Epub 2016 Oct 27. Eur J Clin Microbiol Infect Dis. 2017. PMID: 27787664 Free PMC article.
-
Excitable models: Projections, targets, and the making of futures without disease.Sociol Health Illn. 2021 May;43(4):859-880. doi: 10.1111/1467-9566.13263. Epub 2021 May 4. Sociol Health Illn. 2021. PMID: 33942914 Free PMC article.
-
Cost-effectiveness of Universal Hepatitis C Virus Screening of Pregnant Women in the United States.Clin Infect Dis. 2019 Nov 13;69(11):1888-1895. doi: 10.1093/cid/ciz063. Clin Infect Dis. 2019. PMID: 30689769 Free PMC article.
-
Economic Evaluation of Direct-Acting Antivirals for Hepatitis C in Norway.Pharmacoeconomics. 2018 May;36(5):591-601. doi: 10.1007/s40273-017-0604-3. Pharmacoeconomics. 2018. PMID: 29396744 Free PMC article.
References
-
- Shepard C.W., Finelli L., Alter M.J. Global epidemiology of hepatitis C virus infection. Lancet Infect Dis. 2005;5:558–567. - PubMed
-
- Gower E., Estes C., Blach S., Razavi-Shearer K., Razavi H. Global epidemiology and genotype distribution of the hepatitis C virus infection. J Hepatol. 2014;61:S45–S57. - PubMed
-
- De Angelis D., Sweeting M., Ades A.E., Hickman M., Hope V., Ramsay M. An evidence synthesis approach to estimating Hepatitis C Prevalence in England and Wales. Stat Methods Med Res. 2009;18:361–379. - PubMed
-
- Dore G.J., Feld J. Hepatitis C virus therapeutic development: in pursuit of perfectovir. Clin Infect Dis. 2015;60:1829–1836. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

