Structural Studies of Lipopolysaccharide-defective Mutants from Brucella melitensis Identify a Core Oligosaccharide Critical in Virulence
- PMID: 26867577
- PMCID: PMC4817197
- DOI: 10.1074/jbc.M115.701540
Structural Studies of Lipopolysaccharide-defective Mutants from Brucella melitensis Identify a Core Oligosaccharide Critical in Virulence
Abstract
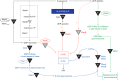
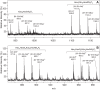
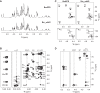
The structures of the lipooligosaccharides fromBrucella melitensismutants affected in the WbkD and ManBcoreproteins have been fully characterized using NMR spectroscopy. The results revealed that disruption ofwbkDgives rise to a rough lipopolysaccharide (R-LPS) with a complete core structure (β-d-Glcp-(1→4)-α-Kdop-(2→4)[β-d-GlcpN-(1→6)-β-d-GlcpN-(1→4)[β-d-GlcpN-(1→6)]-β-d-GlcpN-(1→3)-α-d-Manp-(1→5)]-α-Kdop-(2→6)-β-d-GlcpN3N4P-(1→6)-α-d-GlcpN3N1P), in addition to components lacking one of the terminal β-d-GlcpN and/or the β-d-Glcpresidues (48 and 17%, respectively). These structures were identical to those of the R-LPS fromB. melitensisEP, a strain simultaneously expressing both smooth and R-LPS, also studied herein. In contrast, disruption ofmanBcoregives rise to a deep-rough pentasaccharide core (β-d-Glcp-(1→4)-α-Kdop-(2→4)-α-Kdop-(2→6)-β-d-GlcpN3N4P-(1→6)-α-d-GlcpN3N1P) as the major component (63%), as well as a minor tetrasaccharide component lacking the terminal β-d-Glcpresidue (37%). These results are in agreement with the predicted functions of the WbkD (glycosyltransferase involved in the biosynthesis of the O-antigen) and ManBcoreproteins (phosphomannomutase involved in the biosynthesis of a mannosyl precursor needed for the biosynthesis of the core and O-antigen). We also report that deletion ofB. melitensis wadCremoves the core oligosaccharide branch not linked to the O-antigen causing an increase in overall negative charge of the remaining LPS inner section. This is in agreement with the mannosyltransferase role predicted for WadC and the lack of GlcpN residues in the defective core oligosaccharide. Despite carrying the O-antigen essential inB. melitensisvirulence, the core deficiency in thewadCmutant structure resulted in a more efficient detection by innate immunity and attenuation, proving the role of the β-d-GlcpN-(1→6)-β-d-GlcpN-(1→4)[β-d-GlcpN-(1→6)]-β-d-GlcpN-(1→3)-α-d-Manp-(1→5) structure in virulence.
Keywords: Brucella melitensis; Gram-negative bacteria; WadC; glycosyltransferase; lipopolysaccharide (LPS); mutant; nuclear magnetic resonance (NMR).
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures




References
-
- McDermott J., Grace D., and Zinsstag J. (2013) Economics of brucellosis impact and control in low-income countries. Rev. Sci. Tech. 32, 249–261 - PubMed
-
- Ariza J. (1999) Brucellosis: an update. The perspective from the Mediterranean basin. Rev. Med. Micriobiol. 10, 125–135
-
- Valvano M. A., Furlong S. E., and Patel K. B. (2011) in Bacterial Lipopolysaccharides (Knirel Y. A., and Valvano M. A., eds) pp. 275–310, Springer-Verlag, Wien
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

