Nutlin-3 treatment spares cisplatin-induced inhibition of bone healing while maintaining osteosarcoma toxicity
- PMID: 26867804
- PMCID: PMC5516939
- DOI: 10.1002/jor.23192
Nutlin-3 treatment spares cisplatin-induced inhibition of bone healing while maintaining osteosarcoma toxicity
Abstract
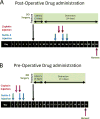
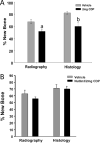
The majority of Osteosarcoma (OS) patients are treated with a combination of chemotherapy, resection, and limb salvage protocols. These protocols include distraction osteogenesis (DO), which is characterized by direct new bone formation. Cisplatin (CDP) is extensively used for OS chemotherapy and recent studies, using a mouse DO model, have demonstrated that CDP has profound negative effects on bone repair. Recent oncological therapeutic strategies are based on the use of standard cytotoxic drugs plus an assortment of biologic agents. Here we demonstrate that the previously reported CDP-associated inhibition of bone repair can be modulated by the administration of a small molecule p53 inducer (nutlin-3). The effects of nutlin-3 on CDP osteotoxicity were studied using both pre- and post-operative treatment models. In both cases the addition of nutlin-3, bracketing CDP exposure, demonstrated robust and significant bone sparing activity (p < 0.01-0.001). In addition the combination of nutlin-3 and CDP induced equivalent OS tumor killing in a xenograft model. Collectively, these results demonstrate that the induction of p53 peri-operatively protects bone healing from the toxic effects of CDP, while maintaining OS toxicity. © 2016 Orthopaedic Research Society. Published by Wiley Periodicals, Inc. J Orthop Res 34:1716-1724, 2016.
Keywords: chemotherapy; cisplatin; distraction osteogenesis; limb salvage; nutlin-3.
© 2016 Orthopaedic Research Society. Published by Wiley Periodicals, Inc.
Figures

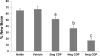

References
-
- Messerschmitt PJ, Garcia RM, Abdul-Karim FW, et al. Osteosarcoma. J Am Acad Orthop Surg. 2009;17:515–527. - PubMed
-
- Bilariki K, Anagnostou E, Masse V. Low bone mineral density and high incidences of fractures and vitamin D deficiency in 52 pediatric cancer survivors. Horm Res Paediatric. 2010;74:319–327. - PubMed
-
- Bertoldo F, Pancheri S, Zenari S, et al. Emerging drugs for the management of cancer treatment induced bone loss. Expert Opin Emerg Drugs. 2010;15:323–342. Review. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

