Comparative immunogenicity and structural analysis of epitopes of different bacterial L-asparaginases
- PMID: 26867931
- PMCID: PMC4750198
- DOI: 10.1186/s12885-016-2125-4
Comparative immunogenicity and structural analysis of epitopes of different bacterial L-asparaginases
Abstract
Background: E.coli type II L-asparaginase is widely used for treatment of acute lymphoblastic leukemia. However, serious side effects such as allergic or hypersensitivity reactions are common for L-asparaginase treatment. Methods for minimizing immune response on L-asparaginase treatment in human include bioengeneering of less immunogenic version of the enzyme or utilizing the homologous enzymes of different origin. To rationalize these approaches we compared immunogenicity of L-asparaginases from five bacterial organisms and performed sequence-structure analysis of the presumable epitope regions.
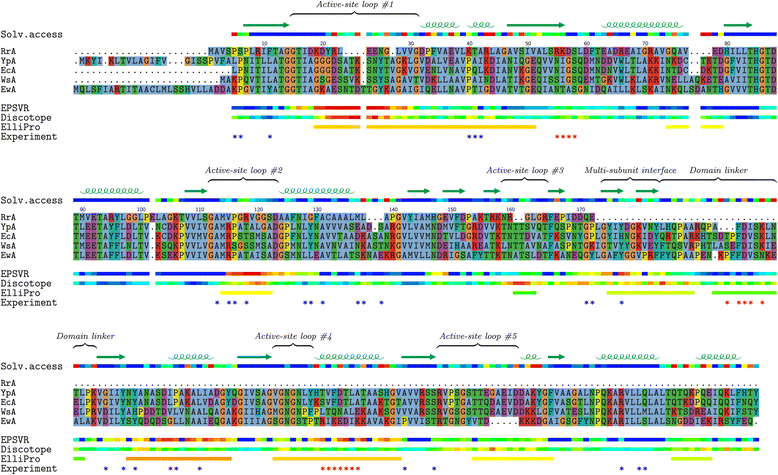
Methods: IgG and IgM immune response in C57B16 mice after immunization with Wollinella succinogenes type II (WsA), Yersinia pseudotuberculosis type II (YpA), Erwinia carotovora type II (EwA), and Rhodospirillum rubrum type I (RrA) and Escherichia coli type II (EcA) L-asparaginases was evaluated using standard ELISA method. The comparative bioinformatics analysis of structure and sequence of the bacterial L-asparaginases presumable epitope regions was performed.
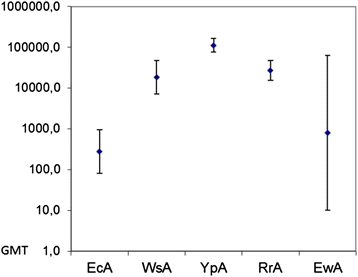
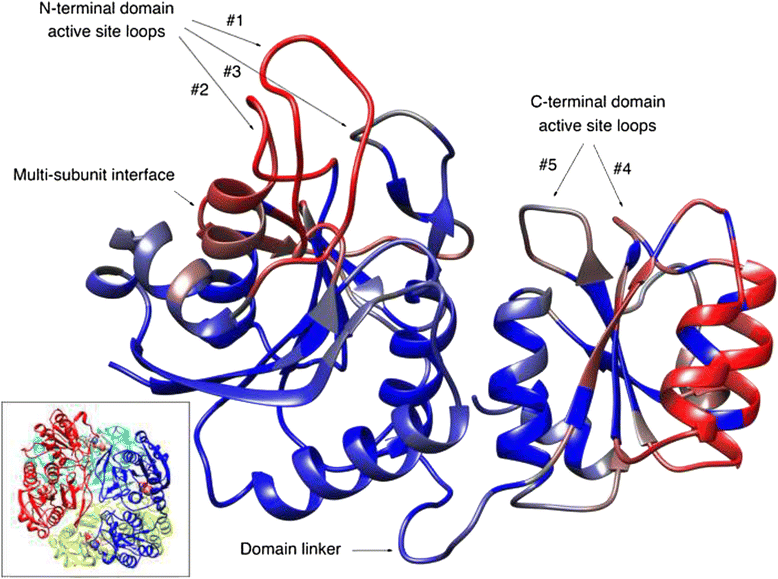
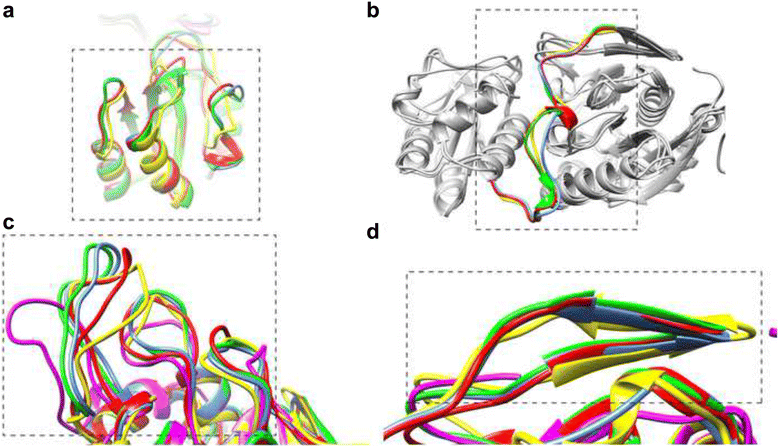
Results: We showed different immunogenic properties of five studied L-asparaginases and confirmed the possibility of replacement of EcA with L-asparaginase from different origin as a second-line treatment. Studied L-asparaginases might be placed in the following order based on the immunogenicity level: YpA > RrA, WsA ≥ EwA > EcA. Most significant cross-immunogenicity was shown between EcA and YpA. We propose that a long N-terminus of YpA enzyme enriched with charged aminoacids and tryptophan could be a reason of higher immunogenicity of YpA in comparison with other considered enzymes. Although the recognized structural and sequence differences in putative epitope regions among five considered L-asparaginases does not fully explain experimental observation of the immunogenicity of the enzymes, the performed analysis set the foundation for further research in this direction.
Conclusions: The performed studies showed different immunogenic properties of L-asparaginases and confirmed the possibility of replacement of EcA with L-asparaginase from different origin. The preferable enzymes for the second line treatment are WsA, RrA, or EwA.
Figures
Similar articles
-
[Cross-immunogenicity of various bacterial L-asparaginases].Zh Mikrobiol Epidemiol Immunobiol. 2014 Nov-Dec;(6):100-4. Zh Mikrobiol Epidemiol Immunobiol. 2014. PMID: 25816523 Russian.
-
[Bacterial recombinant L-asparaginases: properties, structure and anti-proliferative activity].Biomed Khim. 2015 May-Jun;61(3):312-24. doi: 10.18097/PBMC20156103312. Biomed Khim. 2015. PMID: 26215408 Review. Russian.
-
A structural in silico analysis of the immunogenicity of l-asparaginase from Escherichia coli and Erwinia carotovora.Biologicals. 2019 May;59:47-55. doi: 10.1016/j.biologicals.2019.03.003. Epub 2019 Mar 11. Biologicals. 2019. PMID: 30871932
-
[Suppression of telomerase activity leukemic cells by mutant forms of Rhodospirillum rubrum L-asparaginase].Biomed Khim. 2017 Jan;63(1):62-74. doi: 10.18097/PBMC2017630162. Biomed Khim. 2017. PMID: 28251953 Russian.
-
Optimizing asparaginase therapy for acute lymphoblastic leukemia.Curr Opin Oncol. 2013 Mar;25 Suppl 1:S1-9. doi: 10.1097/CCO.0b013e32835d7d85. Curr Opin Oncol. 2013. PMID: 23380829 Review.
Cited by
-
Expression and Biological Evaluation of an Engineered Recombinant L-asparaginase Designed by In Silico Method Based on Sequence of the Enzyme from Escherichia coli.Adv Pharm Bull. 2023 Nov;13(4):827-836. doi: 10.34172/apb.2023.085. Epub 2023 Jun 12. Adv Pharm Bull. 2023. PMID: 38022803 Free PMC article.
-
Penetration into Cancer Cells via Clathrin-Dependent Mechanism Allows L-Asparaginase from Rhodospirillum rubrum to Inhibit Telomerase.Pharmaceuticals (Basel). 2020 Sep 30;13(10):286. doi: 10.3390/ph13100286. Pharmaceuticals (Basel). 2020. PMID: 33008089 Free PMC article.
-
Improvement of Biocatalytic Properties and Cytotoxic Activity of L-Asparaginase from Rhodospirillum rubrum by Conjugation with Chitosan-Based Cationic Polyelectrolytes.Pharmaceuticals (Basel). 2022 Mar 27;15(4):406. doi: 10.3390/ph15040406. Pharmaceuticals (Basel). 2022. PMID: 35455403 Free PMC article.
-
Analgesic effects of nerve growth factor-directed monoclonal antibody on diabetic neuralgia in an animal model.FEBS Open Bio. 2022 Jul;12(7):1325-1335. doi: 10.1002/2211-5463.13410. Epub 2022 May 3. FEBS Open Bio. 2022. PMID: 35417079 Free PMC article.
-
Characterization of Mycobacterium smegmatis Glutaminase-Free Asparaginase (MSMEG_3173).ACS Omega. 2024 Sep 12;9(38):40214-40225. doi: 10.1021/acsomega.4c06459. eCollection 2024 Sep 24. ACS Omega. 2024. PMID: 39346838 Free PMC article.
References
-
- Warrell RP, Jr, Arlin ZA, Gee TS, Chou TC, Roberts J, Young CW. Clinical evaluation of succinylated Acinetobacter glutaminase-asparaginase in adult leukemia. Cancer Treat. Rep. 1982;66(7):1479–1485. - PubMed
-
- Abuchowski A, Kazo GM, Verhoest CR, Jr, Van Es T, Kafkewitz D, Nucci ML, Viau AT, Davis FF. Cancer therapy with chemically modified enzymes. I. Antitumor properties of polyethylene glycol-asparaginase conjugates. Cancer Biochem Biophys. 1984;7(2):175–186. - PubMed
-
- Asselin BL, Whitin JC, Coppola DJ, Rupp IP, Sallan SE, Cohen HJ. Comparative pharmacokinetic studies of three asparaginase preparations. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 1993;11(9):1780–1786. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

