Portal of medical data models: information infrastructure for medical research and healthcare
- PMID: 26868052
- PMCID: PMC4750548
- DOI: 10.1093/database/bav121
Portal of medical data models: information infrastructure for medical research and healthcare
Abstract
Introduction: Information systems are a key success factor for medical research and healthcare. Currently, most of these systems apply heterogeneous and proprietary data models, which impede data exchange and integrated data analysis for scientific purposes. Due to the complexity of medical terminology, the overall number of medical data models is very high. At present, the vast majority of these models are not available to the scientific community. The objective of the Portal of Medical Data Models (MDM, https://medical-data-models.org) is to foster sharing of medical data models.
Methods: MDM is a registered European information infrastructure. It provides a multilingual platform for exchange and discussion of data models in medicine, both for medical research and healthcare. The system is developed in collaboration with the University Library of Münster to ensure sustainability. A web front-end enables users to search, view, download and discuss data models. Eleven different export formats are available (ODM, PDF, CDA, CSV, MACRO-XML, REDCap, SQL, SPSS, ADL, R, XLSX). MDM contents were analysed with descriptive statistics.
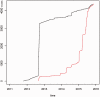
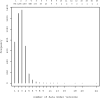
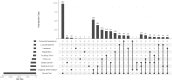
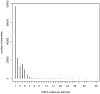
Results: MDM contains 4387 current versions of data models (in total 10,963 versions). 2475 of these models belong to oncology trials. The most common keyword (n = 3826) is 'Clinical Trial'; most frequent diseases are breast cancer, leukemia, lung and colorectal neoplasms. Most common languages of data elements are English (n = 328,557) and German (n = 68,738). Semantic annotations (UMLS codes) are available for 108,412 data items, 2453 item groups and 35,361 code list items. Overall 335,087 UMLS codes are assigned with 21,847 unique codes. Few UMLS codes are used several thousand times, but there is a long tail of rarely used codes in the frequency distribution.
Discussion: Expected benefits of the MDM portal are improved and accelerated design of medical data models by sharing best practice, more standardised data models with semantic annotation and better information exchange between information systems, in particular Electronic Data Capture (EDC) and Electronic Health Records (EHR) systems. Contents of the MDM portal need to be further expanded to reach broad coverage of all relevant medical domains. Database URL: https://medical-data-models.org.
© The Author(s) 2016. Published by Oxford University Press.
Figures



References
-
- AllTrials. http://www.alltrials.net/ (10 August 2015, date last accessed).
-
- SNOMED CT. http://www.ihtsdo.org/snomed-ct/ (10 August 2015, date last accessed, archived at http://www.webcitation.org/6agIgQreb).
-
- ClinicalTrials.gov. http://Clinicaltrials.gov (22 September 2015, date last accessed, archived at http://www.webcitation.org/6bjgR21MO).
-
- Getz K. Protocol Design Trends and their Effect on Clinical Trial Performance. http://csdd.tufts.edu/_documents/www/2816Getz.pdf (10 August 2015, date last accessed, archived at http://www.webcitation.org/6agJILUZv).
-
- International classification of diseases (ICD). http://www.who.int/classifications/icd/en/ (10 August 2015, date last accessed, archived at http://www.webcitation.org/6agIwgGXi).
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

