Red Sea Atlantis II brine pool nitrilase with unique thermostability profile and heavy metal tolerance
- PMID: 26868129
- PMCID: PMC4751646
- DOI: 10.1186/s12896-016-0244-2
Red Sea Atlantis II brine pool nitrilase with unique thermostability profile and heavy metal tolerance
Abstract
Background: Nitrilases, which hydrolyze nitriles in a one-step reaction into carboxylic acids and ammonia, gained increasing attention because of the abundance of nitrile compounds in nature and their use in fine chemicals and pharmaceutics. Extreme environments are potential habitats for the isolation and characterization of extremozymes including nitrilases with unique resistant properties. The Red Sea brine pools are characterized by multitude of extreme conditions. The Lower Convective Layer (LCL) of the Atlantis II Deep Brine Pool in the Red Sea is characterized by elevated temperature (68 °C), high salt concentrations (250 ‰), anoxic conditions and high heavy metal concentrations.
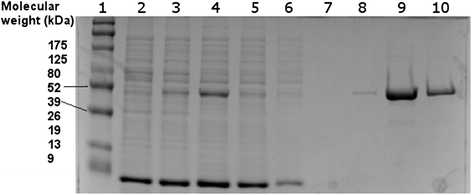
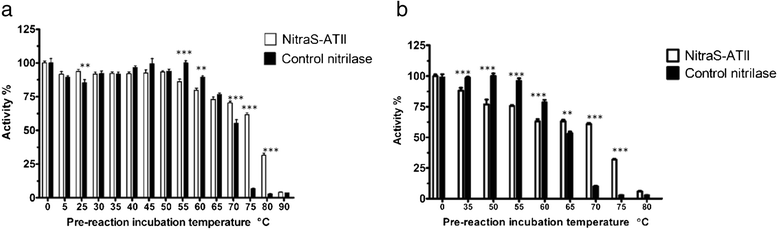
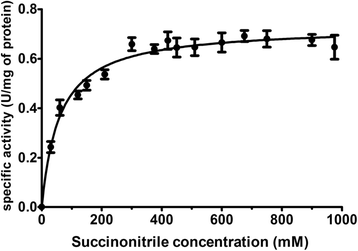
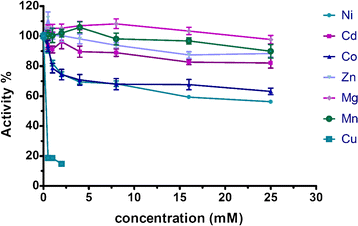
Results: We identified and isolated a nitrilase from the Atlantis II Deep Brine Pool in the Red Sea LCL. The isolated 338 amino-acid nitrilase (NitraS-ATII) is part of a highly conserved operon in different bacterial phyla with indiscernible function. The enzyme was cloned, expressed and purified. Characterization of the purified NitraS-ATII revealed its selectivity towards dinitriles, which suggests a possible industrial application in the synthesis of cyanocarboxylic acids. Moreover, NitraS-ATII showed higher thermal stability compared to a closely related nitrilase, in addition to its observed tolerance towards high concentrations of selected heavy metals.
Conclusion: This enzyme sheds light on evolution of microbes in the Atlantis II Deep LCL to adapt to the diverse extreme environment and can prove to be valuable in bioremediation processes.
Figures



Similar articles
-
Thermal Stability of a Mercuric Reductase from the Red Sea Atlantis II Hot Brine Environment as Analyzed by Site-Directed Mutagenesis.Appl Environ Microbiol. 2019 Jan 23;85(3):e02387-18. doi: 10.1128/AEM.02387-18. Print 2019 Feb 1. Appl Environ Microbiol. 2019. PMID: 30446558 Free PMC article.
-
A novel mercuric reductase from the unique deep brine environment of Atlantis II in the Red Sea.J Biol Chem. 2014 Jan 17;289(3):1675-87. doi: 10.1074/jbc.M113.493429. Epub 2013 Nov 26. J Biol Chem. 2014. PMID: 24280218 Free PMC article.
-
Insertion sequences enrichment in extreme Red sea brine pool vent.Extremophiles. 2017 Mar;21(2):271-282. doi: 10.1007/s00792-016-0900-4. Epub 2016 Dec 3. Extremophiles. 2017. PMID: 27915389
-
Biochemistry and biotechnology of mesophilic and thermophilic nitrile metabolizing enzymes.Extremophiles. 1998 Aug;2(3):207-16. doi: 10.1007/s007920050062. Extremophiles. 1998. PMID: 9783167 Review.
-
Recent advances and challenges in the heterologous production of microbial nitrilases for biocatalytic applications.World J Microbiol Biotechnol. 2017 Jan;33(1):8. doi: 10.1007/s11274-016-2173-6. Epub 2016 Nov 17. World J Microbiol Biotechnol. 2017. PMID: 27858339 Review.
Cited by
-
Insights into Red Sea Brine Pool Specialized Metabolism Gene Clusters Encoding Potential Metabolites for Biotechnological Applications and Extremophile Survival.Mar Drugs. 2019 May 8;17(5):273. doi: 10.3390/md17050273. Mar Drugs. 2019. PMID: 31071993 Free PMC article.
-
Antibacterial and anticancer activities of orphan biosynthetic gene clusters from Atlantis II Red Sea brine pool.Microb Cell Fact. 2019 Mar 18;18(1):56. doi: 10.1186/s12934-019-1103-3. Microb Cell Fact. 2019. PMID: 30885206 Free PMC article.
-
Metagenomics of Thermophiles with a Focus on Discovery of Novel Thermozymes.Front Microbiol. 2016 Sep 27;7:1521. doi: 10.3389/fmicb.2016.01521. eCollection 2016. Front Microbiol. 2016. PMID: 27729905 Free PMC article. Review.
-
Metagenomic profiling of antibiotic resistance genes in Red Sea brine pools.Arch Microbiol. 2023 Apr 15;205(5):195. doi: 10.1007/s00203-023-03531-x. Arch Microbiol. 2023. PMID: 37061654
-
Novel thermostable antibiotic resistance enzymes from the Atlantis II Deep Red Sea brine pool.Microb Biotechnol. 2017 Jan;10(1):189-202. doi: 10.1111/1751-7915.12468. Epub 2016 Dec 22. Microb Biotechnol. 2017. PMID: 28004885 Free PMC article.
References
-
- Kaul P, Banerjee A, Banerjee UC. Nitrile hydrolases. In: Polaina J, MacCabe AP, editors. Industrial enzymes. Netherlands: Springer; 2007. pp. 531–547.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

