Cerebral functional connectivity and Mayer waves in mice: Phenomena and separability
- PMID: 26868180
- PMCID: PMC5381445
- DOI: 10.1177/0271678X16629977
Cerebral functional connectivity and Mayer waves in mice: Phenomena and separability
Abstract
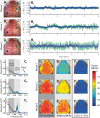
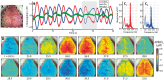
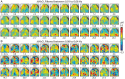
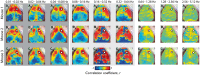
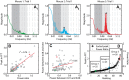
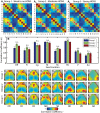
Resting-state functional connectivity is a growing neuroimaging approach that analyses the spatiotemporal structure of spontaneous brain activity, often using low-frequency (<0.08 Hz) hemodynamics. In addition to these fluctuations, there are two other low-frequency hemodynamic oscillations in a nearby spectral region (0.1-0.4 Hz) that have been reported in the brain: vasomotion and Mayer waves. Despite how close in frequency these phenomena exist, there is little research on how vasomotion and Mayer waves are related to or affect resting-state functional connectivity. In this study, we analyze spontaneous hemodynamic fluctuations over the mouse cortex using optical intrinsic signal imaging. We found spontaneous occurrence of oscillatory hemodynamics ∼0.2 Hz consistent with the properties of Mayer waves reported in the literature. Across a group of mice (n = 19), there was a large variability in the magnitude of Mayer waves. However, regardless of the magnitude of Mayer waves, functional connectivity patterns could be recovered from hemodynamic signals when filtered to the lower frequency band, 0.01-0.08 Hz. Our results demonstrate that both Mayer waves and resting-state functional connectivity patterns can co-exist simultaneously, and that they can be separated by applying bandpass filters.
Keywords: Brain imaging; cerebral hemodynamics; cortical mapping; intrinsic optical imaging; neurovascular coupling.
Figures

References
-
- De Luca M, Smith S, De Stefano N, et al. Blood oxygenation level dependent contrast resting state networks are relevant to functional activity in the neocortical sensorimotor system. Exper Brain Res 2005; 167: 587–594. - PubMed
-
- Biswal B, Yetkin FZ, Haughton VM, et al. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med 1995; 34: 537–541. - PubMed
-
- Vincent JL, Patel GH, Fox MD, et al. Intrinsic functional architecture in the anaesthetized monkey brain. Nature 2007; 447: 83–86. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

