Profound Actions of an Agonist of Growth Hormone-Releasing Hormone on Angiogenic Therapy by Mesenchymal Stem Cells
- PMID: 26868211
- PMCID: PMC4808467
- DOI: 10.1161/ATVBAHA.116.307126
Profound Actions of an Agonist of Growth Hormone-Releasing Hormone on Angiogenic Therapy by Mesenchymal Stem Cells
Abstract
Objective: The efficiency of cell therapy is limited by poor cell survival and engraftment. Here, we studied the effect of the growth hormone-releasing hormone agonist, JI-34, on mesenchymal stem cell (MSC) survival and angiogenic therapy in a mouse model of critical limb ischemia.
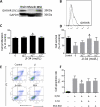
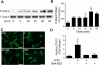
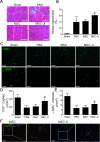
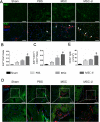
Approach and results: Mouse bone marrow-derived MSCs were incubated with or without 10(-8) mol/L JI-34 for 24 hours. MSCs were then exposed to hypoxia and serum deprivation to detect the effect of preconditioning on cell apoptosis, migration, and tube formation. For in vivo tests, critical limb ischemia was induced by femoral artery ligation. After surgery, mice received 50 μL phosphate-buffered saline or with 1×10(6) MSCs or with 1×10(6) JI-34-reconditioned MSCs. Treatment of MSCs with JI-34 improved MSC viability and mobility and markedly enhanced their capability to promote endothelial tube formation in vitro. These effects were paralleled by an increased phosphorylation and nuclear translocation of signal transducer and activator of transcription 3. In vivo, JI-34 pretreatment enhanced the engraftment of MSCs into ischemic hindlimb muscles and augmented reperfusion and limb salvage compared with untreated MSCs. Significantly more vasculature and proliferating CD31(+) and CD34(+) cells were detected in ischemic muscles that received MSCs treated with JI-34.
Conclusions: Our studies demonstrate a novel role for JI-34 to markedly improve therapeutic angiogenesis in hindlimb ischemia by increasing the viability and mobility of MSCs. These findings support additional studies to explore the full potential of growth hormone-releasing hormone agonists to augment cell therapy in the management of ischemia.
Keywords: angiogenesis effects; growth hormone–releasing hormone; mesenchymal stromal cells.
© 2016 American Heart Association, Inc.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

