Lipids and immunoinflammatory pathways of beta cell destruction
- PMID: 26868492
- PMCID: PMC4779407
- DOI: 10.1007/s00125-016-3890-y
Lipids and immunoinflammatory pathways of beta cell destruction
Abstract
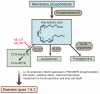
Islet inflammation contributes to beta cell demise in both type 1 and type 2 diabetes. 12-Lipoxygenase (12-LO, gene expressed as ALOX12 in humans and 12-Lo in rodents in this manuscript) produces proinflammatory metabolites such as 12(S)-hydroxyeicosatetraenoic acids through dioxygenation of polyunsaturated fatty acids. 12-LO was first implicated in diabetes when the increase in 12-Lo expression and 12(S)-hydroxyeicosatetraenoic acid was noted in rodent models of diabetes. Subsequently, germline 12-Lo (-/-) was shown to prevent the development of hyperglycemia in mouse models of type 1 diabetes and in high-fat fed mice. More recently, beta cell-specific 12-Lo (-/-) was shown to protect mice against hyperglycaemia after streptozotocin and a high-fat diet. In humans, 12-LO expression is increased in pancreatic islets of autoantibody-positive, type 1 diabetic and type 2 diabetic organ donors. Interestingly, the high expression of ALOX12 is associated with the alteration in first-phase glucose-stimulated insulin secretion in human type 2 diabetic islets. To further clarify the role of islet 12-LO in diabetes and to validate 12-LO as a therapeutic target of diabetes, we have studied selective pharmacological inhibitors for 12-LO. The compounds we have identified show promise: they protect beta cell lines and human islets from apoptosis and preserve insulin secretion when challenged by proinflammatory cytokine mixture. Currently studies are underway to test the compounds in mouse models of diabetes. This review summarises a presentation given at the 'Islet inflammation in type 2 diabetes' symposium at the 2015 annual meeting of the EASD. It is accompanied two other mini-reviews on topics from this symposium (by Simone Baltrusch, DOI: 10.1007/s00125-016-3891-x and Marc Donath, DOI: 10.1007/s00125-016-3873-z ) and a commentary by the Session Chair, Piero Marchetti (DOI: 10.1007/s00125-016-3875-x ).
Keywords: 12(S)-Hydroxyeicosatetraenoic acid; 12-Lipoxygenase; Review; Small molecule inhibitors; Type 1 diabetes; Type 2 diabetes.
Figures
Similar articles
-
Expression pattern of 12-lipoxygenase in human islets with type 1 diabetes and type 2 diabetes.J Clin Endocrinol Metab. 2015 Mar;100(3):E387-95. doi: 10.1210/jc.2014-3630. Epub 2014 Dec 22. J Clin Endocrinol Metab. 2015. PMID: 25532042 Free PMC article.
-
Multiple benefits of targeting inflammation in the treatment of type 2 diabetes.Diabetologia. 2016 Apr;59(4):679-82. doi: 10.1007/s00125-016-3873-z. Epub 2016 Feb 11. Diabetologia. 2016. PMID: 26868493 Review.
-
MST1: a promising therapeutic target to restore functional beta cell mass in diabetes.Diabetologia. 2016 Sep;59(9):1843-9. doi: 10.1007/s00125-016-3892-9. Epub 2016 Apr 6. Diabetologia. 2016. PMID: 27053234 Review.
-
Integration of pro-inflammatory cytokines, 12-lipoxygenase and NOX-1 in pancreatic islet beta cell dysfunction.Mol Cell Endocrinol. 2012 Jul 6;358(1):88-95. doi: 10.1016/j.mce.2012.03.004. Epub 2012 Mar 20. Mol Cell Endocrinol. 2012. PMID: 22502743
-
Mitochondrial network regulation and its potential interference with inflammatory signals in pancreatic beta cells.Diabetologia. 2016 Apr;59(4):683-7. doi: 10.1007/s00125-016-3891-x. Epub 2016 Feb 12. Diabetologia. 2016. PMID: 26873508 Review.
Cited by
-
Islet inflammation in type 2 diabetes.Diabetologia. 2016 Apr;59(4):668-72. doi: 10.1007/s00125-016-3875-x. Epub 2016 Feb 19. Diabetologia. 2016. PMID: 26894276 Review. No abstract available.
-
Synopsis of arachidonic acid metabolism: A review.J Adv Res. 2018 Mar 13;11:23-32. doi: 10.1016/j.jare.2018.03.005. eCollection 2018 May. J Adv Res. 2018. PMID: 30034873 Free PMC article. Review.
-
Restoring connexin-36 function in diabetogenic environments precludes mouse and human islet dysfunction.J Physiol. 2023 Sep;601(18):4053-4072. doi: 10.1113/JP282114. Epub 2023 Aug 14. J Physiol. 2023. PMID: 37578890 Free PMC article.
-
Macrophage polarization is linked to Ca2+-independent phospholipase A2β-derived lipids and cross-cell signaling in mice.J Lipid Res. 2020 Feb;61(2):143-158. doi: 10.1194/jlr.RA119000281. Epub 2019 Dec 9. J Lipid Res. 2020. PMID: 31818877 Free PMC article.
-
Pancreas Pathology During the Natural History of Type 1 Diabetes.Curr Diab Rep. 2018 Oct 6;18(11):124. doi: 10.1007/s11892-018-1084-3. Curr Diab Rep. 2018. PMID: 30293191 Review.
References
-
- Richardson SJ, Willcox A, Bone AJ, Foulis AK, Morgan NG. Islet-associated macrophages in type 2 diabetes. Diabetologia. 2009;52:1686–1688. - PubMed
-
- Westwell-Roper CY, Ehses JA, Verchere CB. Resident macrophages mediate islet amyloid polypeptide-induced islet IL-1beta production and beta-cell dysfunction. Diabetes. 2014;63:1698–1711. - PubMed
-
- Eguchi K, Manabe I, Oishi-Tanaka Y, et al. Saturated fatty acid and TLR signaling link beta cell dysfunction and islet inflammation. Cell metabolism. 2012;15:518–533. - PubMed
-
- Westwell-Roper C, Nackiewicz D, Dan M, Ehses JA. Toll-like receptors and NLRP3 as central regulators of pancreatic islet inflammation in type 2 diabetes. Immunology and cell biology. 2014;92:314–323. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

