Fate decision of mesenchymal stem cells: adipocytes or osteoblasts?
- PMID: 26868907
- PMCID: PMC4946886
- DOI: 10.1038/cdd.2015.168
Fate decision of mesenchymal stem cells: adipocytes or osteoblasts?
Abstract
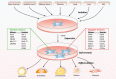
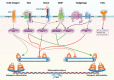
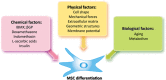
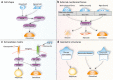
Mesenchymal stem cells (MSCs), a non-hematopoietic stem cell population first discovered in bone marrow, are multipotent cells capable of differentiating into mature cells of several mesenchymal tissues, such as fat and bone. As common progenitor cells of adipocytes and osteoblasts, MSCs are delicately balanced for their differentiation commitment. Numerous in vitro investigations have demonstrated that fat-induction factors inhibit osteogenesis, and, conversely, bone-induction factors hinder adipogenesis. In fact, a variety of external cues contribute to the delicate balance of adipo-osteogenic differentiation of MSCs, including chemical, physical, and biological factors. These factors trigger different signaling pathways and activate various transcription factors that guide MSCs to commit to either lineage. The dysregulation of the adipo-osteogenic balance has been linked to several pathophysiologic processes, such as aging, obesity, osteopenia, osteopetrosis, and osteoporosis. Thus, the regulation of MSC differentiation has increasingly attracted great attention in recent years. Here, we review external factors and their signaling processes dictating the reciprocal regulation between adipocytes and osteoblasts during MSC differentiation and the ultimate control of the adipo-osteogenic balance.
Figures
References
-
- Teitelbaum SL. Bone resorption by osteoclasts. Science 2000; 289: 1504–1508. - PubMed
-
- Caplan AI. Mesenchymal stem-cells. J Orthop Res 1991; 9: 641–650. - PubMed
-
- Horwitz EM, Le Blanc K, Dominici M, Mueller I, Slaper-Cortenbach I, Marini FC et al. Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy 2005; 7: 393–395. - PubMed
-
- Horwitz EM, Prockop DJ, Fitzpatrick LA, Koo WWK, Gordon PL, Neel M et al. Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat Med 1999; 5: 309–313. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

