Inflammasome-dependent IL-1β release depends upon membrane permeabilisation
- PMID: 26868913
- PMCID: PMC4946890
- DOI: 10.1038/cdd.2015.176
Inflammasome-dependent IL-1β release depends upon membrane permeabilisation
Abstract
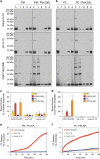
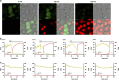
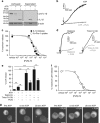
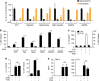
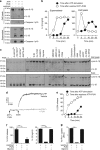
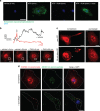
Interleukin-1β (IL-1β) is a critical regulator of the inflammatory response. IL-1β is not secreted through the conventional ER-Golgi route of protein secretion, and to date its mechanism of release has been unknown. Crucially, its secretion depends upon the processing of a precursor form following the activation of the multimolecular inflammasome complex. Using a novel and reversible pharmacological inhibitor of the IL-1β release process, in combination with biochemical, biophysical, and real-time single-cell confocal microscopy with macrophage cells expressing Venus-labelled IL-1β, we have discovered that the secretion of IL-1β after inflammasome activation requires membrane permeabilisation, and occurs in parallel with the death of the secreting cell. Thus, in macrophages the release of IL-1β in response to inflammasome activation appears to be a secretory process independent of nonspecific leakage of proteins during cell death. The mechanism of membrane permeabilisation leading to IL-1β release is distinct from the unconventional secretory mechanism employed by its structural homologues fibroblast growth factor 2 (FGF2) or IL-1α, a process that involves the formation of membrane pores but does not result in cell death. These discoveries reveal key processes at the initiation of an inflammatory response and deliver new insights into the mechanisms of protein release.
Conflict of interest statement
MP is an employee of Probelte Biotechnology S.L. The other authors declare no conflict of interest.
Figures

References
-
- Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell 2010; 140: 805–820. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

