Dynamic mesolimbic dopamine signaling during action sequence learning and expectation violation
- PMID: 26869075
- PMCID: PMC4751524
- DOI: 10.1038/srep20231
Dynamic mesolimbic dopamine signaling during action sequence learning and expectation violation
Abstract
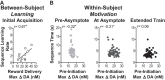
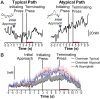
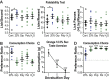
Prolonged mesolimbic dopamine concentration changes have been detected during spatial navigation, but little is known about the conditions that engender this signaling profile or how it develops with learning. To address this, we monitored dopamine concentration changes in the nucleus accumbens core of rats throughout acquisition and performance of an instrumental action sequence task. Prolonged dopamine concentration changes were detected that ramped up as rats executed each action sequence and declined after earned reward collection. With learning, dopamine concentration began to rise increasingly earlier in the execution of the sequence and ultimately backpropagated away from stereotyped sequence actions, becoming only transiently elevated by the most distal and unexpected reward predictor. Action sequence-related dopamine signaling was reactivated in well-trained rats if they became disengaged in the task and in response to an unexpected change in the value, but not identity of the earned reward. Throughout training and test, dopamine signaling correlated with sequence performance. These results suggest that action sequences can engender a prolonged mode of dopamine signaling in the nucleus accumbens core and that such signaling relates to elements of the motivation underlying sequence execution and is dynamic with learning, overtraining and violations in reward expectation.
Figures





References
-
- Schultz W. Getting formal with dopamine and reward. Neuron 36, 241–263 (2002). - PubMed
-
- Waelti P., Dickinson A. & Schultz W. Dopamine responses comply with basic assumptions of formal learning theory. Nature 412, 43–48 (2001). - PubMed
-
- Day J. J., Roitman M. F., Wightman R. M. & Carelli R. M. Associative learning mediates dynamic shifts in dopamine signaling in the nucleus accumbens. Nat Neurosci 10, 1020–1028 (2007). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

