Loss of UBE3A from TH-expressing neurons suppresses GABA co-release and enhances VTA-NAc optical self-stimulation
- PMID: 26869263
- PMCID: PMC4754338
- DOI: 10.1038/ncomms10702
Loss of UBE3A from TH-expressing neurons suppresses GABA co-release and enhances VTA-NAc optical self-stimulation
Abstract
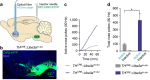
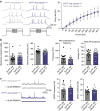
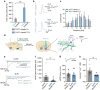
Motivated reward-seeking behaviours are governed by dopaminergic ventral tegmental area projections to the nucleus accumbens. In addition to dopamine, these mesoaccumbal terminals co-release other neurotransmitters including glutamate and GABA, whose roles in regulating motivated behaviours are currently being investigated. Here we demonstrate that loss of the E3-ubiquitin ligase, UBE3A, from tyrosine hydroxylase-expressing neurons impairs mesoaccumbal, non-canonical GABA co-release and enhances reward-seeking behaviour measured by optical self-stimulation.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

