Safety of artemisinins in first trimester of prospectively followed pregnancies: an observational study
- PMID: 26869377
- PMCID: PMC4835584
- DOI: 10.1016/S1473-3099(15)00547-2
Safety of artemisinins in first trimester of prospectively followed pregnancies: an observational study
Erratum in
-
Corrections.Lancet Infect Dis. 2016 May;16(5):521. doi: 10.1016/S1473-3099(16)30014-7. Epub 2016 Apr 18. Lancet Infect Dis. 2016. PMID: 27599646 Free PMC article. No abstract available.
Abstract
Background: Artemisinins, the most effective antimalarials available, are not recommended for falciparum malaria during the first trimester of pregnancy because of safety concerns. Therefore, quinine is used despite its poor effectiveness. Assessing artemisinin safety requires weighing the risks of malaria and its treatment. We aimed to assess the effect of first-trimester malaria and artemisinin treatment on miscarriage and major congenital malformations.
Methods: In this observational study, we assessed data from antenatal clinics on the Thai-Myanmar border between Jan 1, 1994, and Dec 31, 2013. We included women who presented to antenatal clinics during their first trimester with a viable fetus. Women were screened for malaria, and data on malaria, antimalarial treatment, and birth outcomes were collected. The relationship between artemisinin treatments (artesunate, dihydroartemisinin, or artemether) and miscarriage or malformation was assessed using Cox regression with left-truncation and time-varying exposures.
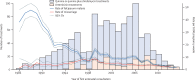
Findings: Of 55 636 pregnancies registered between 1994 and 2013, 25 485 pregnancies were analysed for first-trimester malaria and miscarriage, in which 2558 (10%) had first-trimester malaria. The hazard of miscarriage increased 1·61-fold after an initial first-trimester falciparum episode (95% CI 1·32-1·97; p<0·0001), 3·24-fold following falciparum recurrence (2·24-4·68; p<0·0001), and 2·44-fold (1·01-5·88; p=0·0473) following recurrent symptomatic vivax malaria. No difference was noted in miscarriage in first-line falciparum treatments with artemisinin (n=183) versus quinine (n=842; HR 0·78 [95% CI 0·45-1·34]; p=0·3645) or in risk of major congenital malformations (two [2%] of 109 [95% CI 0·22-6·47] versus eight (1%) of 641 [0·54-2·44], respectively).
Interpretation: First-trimester falciparum and vivax malaria both increase the risk of miscarriage. We noted no evidence of an increased risk of miscarriage or of major congenital malformations associated with first-line treatment with an artemisinin derivative compared with quinine. In view of the low efficacy of quinine and wide availability of highly effective artemisinin-based combination therapies, it is time to reconsider first-trimester antimalarial treatment recommendations.
Funding: The Wellcome Trust and The Bill & Melinda Gates Foundation.
Copyright © 2016 Moore et al. Open Access article distributed under the terms of CC BY. Published by Elsevier Ltd.. All rights reserved.
Figures


Comment in
-
Mounting evidence for use of artemisinin derivatives for malaria in early pregnancy.Lancet Infect Dis. 2016 May;16(5):513-515. doi: 10.1016/S1473-3099(16)00019-0. Epub 2016 Feb 8. Lancet Infect Dis. 2016. PMID: 26869379 No abstract available.
Similar articles
-
First-trimester artemisinin derivatives and quinine treatments and the risk of adverse pregnancy outcomes in Africa and Asia: A meta-analysis of observational studies.PLoS Med. 2017 May 2;14(5):e1002290. doi: 10.1371/journal.pmed.1002290. eCollection 2017 May. PLoS Med. 2017. PMID: 28463996 Free PMC article.
-
Pregnancy outcomes after first-trimester treatment with artemisinin derivatives versus non-artemisinin antimalarials: a systematic review and individual patient data meta-analysis.Lancet. 2023 Jan 14;401(10371):118-130. doi: 10.1016/S0140-6736(22)01881-5. Epub 2022 Nov 25. Lancet. 2023. PMID: 36442488 Free PMC article.
-
Adverse effects of falciparum and vivax malaria and the safety of antimalarial treatment in early pregnancy: a population-based study.Lancet Infect Dis. 2012 May;12(5):388-96. doi: 10.1016/S1473-3099(11)70339-5. Epub 2011 Dec 12. Lancet Infect Dis. 2012. PMID: 22169409 Free PMC article.
-
A randomized controlled trial of dihydroartemisinin-piperaquine, artesunate-mefloquine and extended artemether-lumefantrine treatments for malaria in pregnancy on the Thailand-Myanmar border.BMC Med. 2021 Jun 10;19(1):132. doi: 10.1186/s12916-021-02002-8. BMC Med. 2021. PMID: 34107963 Free PMC article. Clinical Trial.
-
Efficacy and tolerability of artemisinin-based and quinine-based treatments for uncomplicated falciparum malaria in pregnancy: a systematic review and individual patient data meta-analysis.Lancet Infect Dis. 2020 Aug;20(8):943-952. doi: 10.1016/S1473-3099(20)30064-5. Epub 2020 Apr 29. Lancet Infect Dis. 2020. PMID: 32530424 Free PMC article.
Cited by
-
Clinical management of Plasmodium knowlesi malaria.Adv Parasitol. 2021;113:45-76. doi: 10.1016/bs.apar.2021.08.004. Epub 2021 Sep 1. Adv Parasitol. 2021. PMID: 34620385 Free PMC article. Review.
-
The Vivax Surveyor: Online mapping database for Plasmodium vivax clinical trials.Int J Parasitol Drugs Drug Resist. 2017 Aug;7(2):181-190. doi: 10.1016/j.ijpddr.2017.03.003. Epub 2017 Mar 24. Int J Parasitol Drugs Drug Resist. 2017. PMID: 28384505 Free PMC article.
-
Mathematical Modelling to Guide Drug Development for Malaria Elimination.Trends Parasitol. 2017 Mar;33(3):175-184. doi: 10.1016/j.pt.2016.09.004. Epub 2016 Oct 7. Trends Parasitol. 2017. PMID: 27727128 Free PMC article. Review.
-
Influence of the number and timing of malaria episodes during pregnancy on prematurity and small-for-gestational-age in an area of low transmission.BMC Med. 2017 Jun 21;15(1):117. doi: 10.1186/s12916-017-0877-6. BMC Med. 2017. PMID: 28633672 Free PMC article.
-
First-trimester artemisinin derivatives and quinine treatments and the risk of adverse pregnancy outcomes in Africa and Asia: A meta-analysis of observational studies.PLoS Med. 2017 May 2;14(5):e1002290. doi: 10.1371/journal.pmed.1002290. eCollection 2017 May. PLoS Med. 2017. PMID: 28463996 Free PMC article.
References
-
- Huynh B-T, Cottrell G, Cot M, Briand V. Burden of malaria in early pregnancy: a neglected problem? Clin Infect Dis. 2015;60:598–604. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

