Cytosolic calcium transients are a determinant of contraction-induced HSP72 transcription in single skeletal muscle fibers
- PMID: 26869714
- PMCID: PMC4867320
- DOI: 10.1152/japplphysiol.01060.2015
Cytosolic calcium transients are a determinant of contraction-induced HSP72 transcription in single skeletal muscle fibers
Abstract
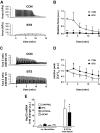
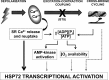
The intrinsic activating factors that induce transcription of heat shock protein 72 (HSP72) in skeletal muscle following exercise remain unclear. We hypothesized that the cytosolic Ca(2+) transient that occurs with depolarization is a determinant. We utilized intact, single skeletal muscle fibers from Xenopus laevis to test the role of the cytosolic Ca(2+) transient and several other exercise-related factors (fatigue, hypoxia, AMP kinase, and cross-bridge cycling) on the activation of HSP72 transcription. HSP72 and HSP60 mRNA levels were assessed with real-time quantitative PCR; cytosolic Ca(2+) concentration ([Ca(2+)]cyt) was assessed with fura-2. Both fatiguing and nonfatiguing contractions resulted in a significant increase in HSP72 mRNA. As expected, peak [Ca(2+)]cyt remained tightly coupled with peak developed tension in contracting fibers. Pretreatment with N-benzyl-p-toluene sulfonamide (BTS) resulted in depressed peak developed tension with stimulation, while peak [Ca(2+)]cyt remained largely unchanged from control values. Despite excitation-contraction uncoupling, BTS-treated fibers displayed a significant increase in HSP72 mRNA. Treatment of fibers with hypoxia (Po2: <3 mmHg) or AMP kinase activation had no effect on HSP72 mRNA levels. These results suggest that the intermittent cytosolic Ca(2+) transient that occurs with skeletal muscle depolarization provides a sufficient activating stimulus for HSP72 transcription. Metabolic or mechanical factors associated with fatigue development and cross-bridge cycling likely play a more limited role.
Keywords: ATP; Ca2+; HSP70; O2; RTq-PCR; exercise; fatigue; gene.
Copyright © 2016 the American Physiological Society.
Figures

Similar articles
-
Measurement of activation energy and oxidative phosphorylation onset kinetics in isolated muscle fibers in the absence of cross-bridge cycling.Am J Physiol Regul Integr Comp Physiol. 2006 Jun;290(6):R1707-13. doi: 10.1152/ajpregu.00687.2005. Epub 2006 Jan 19. Am J Physiol Regul Integr Comp Physiol. 2006. PMID: 16424084
-
Elevation in heat shock protein 72 mRNA following contractions in isolated single skeletal muscle fibers.Am J Physiol Regul Integr Comp Physiol. 2008 Aug;295(2):R642-8. doi: 10.1152/ajpregu.00852.2007. Epub 2008 Jun 4. Am J Physiol Regul Integr Comp Physiol. 2008. PMID: 18525012 Free PMC article.
-
Ca²⁺-pumping impairment during repetitive fatiguing contractions in single myofibers: role of cross-bridge cycling.Am J Physiol Regul Integr Comp Physiol. 2013 Jul 15;305(2):R118-25. doi: 10.1152/ajpregu.00178.2013. Epub 2013 May 15. Am J Physiol Regul Integr Comp Physiol. 2013. PMID: 23678027 Free PMC article.
-
Muscle mechanics: adaptations with exercise-training.Exerc Sport Sci Rev. 1996;24:427-73. Exerc Sport Sci Rev. 1996. PMID: 8744258 Review.
-
Energy turnover for Ca2+ cycling in skeletal muscle.J Muscle Res Cell Motil. 2007;28(4-5):259-74. doi: 10.1007/s10974-007-9116-7. Epub 2007 Sep 20. J Muscle Res Cell Motil. 2007. PMID: 17882515 Review.
Cited by
-
The skeletal muscle fiber: a mechanically sensitive cell.Eur J Appl Physiol. 2019 Feb;119(2):333-349. doi: 10.1007/s00421-018-04061-x. Epub 2019 Jan 5. Eur J Appl Physiol. 2019. PMID: 30612167 Review.
-
Hsp72 and Hsp90α mRNA transcription is characterised by large, sustained changes in core temperature during heat acclimation.Cell Stress Chaperones. 2016 Nov;21(6):1021-1035. doi: 10.1007/s12192-016-0726-0. Epub 2016 Aug 11. Cell Stress Chaperones. 2016. PMID: 27511024 Free PMC article.
-
The effects and post-exercise energy metabolism characteristics of different high-intensity interval training in obese adults.Sci Rep. 2025 Apr 21;15(1):13770. doi: 10.1038/s41598-025-98590-z. Sci Rep. 2025. PMID: 40259013 Free PMC article.
-
Cross-Adaptation: Heat and Cold Adaptation to Improve Physiological and Cellular Responses to Hypoxia.Sports Med. 2017 Sep;47(9):1751-1768. doi: 10.1007/s40279-017-0717-z. Sports Med. 2017. PMID: 28389828 Free PMC article. Review.
References
-
- Abruzzo PM, Esposito F, Marchionni C, di Tullio S, Belia S, Fulle S, Veicsteinas A, Marini M. Moderate exercise training induces ROS-related adaptations to skeletal muscles. Int J Sports Med 34: 676–687, 2013. - PubMed
-
- Amorim FT, Yamada PM, Robergs RA, Schneider SM, Moseley PL. The effect of the rate of heat storage on serum heat shock protein 72 in humans. Eur J Appl Physiol 104: 965–972, 2008. - PubMed
-
- Barash IA, Mathew L, Ryan AF, Chen J, Lieber RL. Rapid muscle-specific gene expression changes after a single bout of eccentric contractions in the mouse. Am J Physiol Cell Physiol 286: C355–C364, 2004. - PubMed
-
- Breen EC, Johnson EC, Wagner H, Tseng HM, Sung LA, Wagner PD. Angiogenic growth factor mRNA responses in muscle to a single bout of exercise. J Appl Physiol 81: 355–361, 1996. - PubMed
-
- Carmeli E, Beiker R, Maor M, Kodesh E. Increased iNOS, MMP-2, and HSP-72 in skeletal muscle following high-intensity exercise training. J Basic Clin Physiol Pharmacol 21: 127–146, 2010. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

