Amelioration of concanavalin A-induced autoimmune hepatitis by magnesium isoglycyrrhizinate through inhibition of CD4(+)CD25(-)CD69(+) subset proliferation
- PMID: 26869766
- PMCID: PMC4734720
- DOI: 10.2147/DDDT.S92440
Amelioration of concanavalin A-induced autoimmune hepatitis by magnesium isoglycyrrhizinate through inhibition of CD4(+)CD25(-)CD69(+) subset proliferation
Abstract
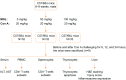
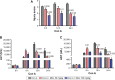
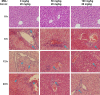
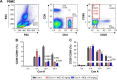
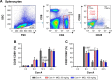
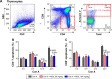
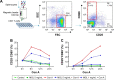
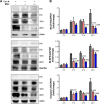
Magnesium isoglycyrrhizinate (MGL) is a new stereoisomer of glycyrrhizic acid, which is clinically used as a hepatoprotective medicine with more potent effects and less side effects than glycyrrhizic acid. This study was designed to evaluate the protective effects and possible mechanism of MGL against concanavalin A (Con A)-induced autoimmune hepatitis. Hepatitis was induced by Con A in C57/6J mice with or without MGL administration; injury score and serum ALT were evaluated. The CD4(+) T-cells were isolated from splenocytes and challenged with Con A after coculturing with MGL. The injury score was significantly improved in MGL-treated mice after Con A challenging for 12 and 24 hours compared with those merely challenged with Con A. Similar trends were observed in the serum levels of ALT and AST. The most interesting result was that MGL administration significantly decreased the frequency of CD4(+)CD25(-)CD69(+) T-cells rather than CD4(+)CD25(+)CD69(+) T-cells in peripheral blood mononuclear cells, after Con A challenging 12 and 24 hours. Moreover, the serum ALT levels were markedly correlated with the frequency of CD4(+)CD25(-)CD69(+) cells, but only weakly correlated with CD4(+)CD25(+)CD69(+) cells in peripheral blood mononuclear cells. More importantly, MGL (5 mg/mL) almost completely eliminated the proliferation of the CD25(-)CD69(+) subset in primary CD4(+) T-cells after Con A challenge. Compared with merely Con A-challenged mice, those with MGL administration significantly demonstrated decreased NALP3, NLRP6, and caspase-3 expression, in which the NALP3 and caspase-3 downregulated in a dose-dependent manner. Our results indicate that MGL may have potential as a therapeutic agent in autoimmune hepatitis by ameliorating liver injury. Its molecular mechanism may be involved in inhibiting CD4(+)CD25(-)CD69(+) subset proliferation and downregulating inflammasome expression in liver tissue.
Keywords: adaptive immunity; autoimmune hepatitis; concanavalin A; drug therapy; mouse; regulatory T-cell.
Figures
Similar articles
-
Glt25d2 Knockout Directly Increases CD25+CD69- but Decreases CD25-CD69+ Subset Proliferation and is Involved in Concanavalin-Induced Hepatitis.Cell Physiol Biochem. 2018;50(3):1186-1200. doi: 10.1159/000494546. Epub 2018 Oct 24. Cell Physiol Biochem. 2018. PMID: 30355948
-
Diammonium Glycyrrhizinate Mitigates Liver Injury Via Inhibiting Proliferation Of NKT Cells And Promoting Proliferation Of Tregs.Drug Des Devel Ther. 2019 Oct 16;13:3579-3589. doi: 10.2147/DDDT.S220030. eCollection 2019. Drug Des Devel Ther. 2019. PMID: 31802846 Free PMC article.
-
CD4+CD69+ T cells and CD4+CD25+FoxP3+ Treg cells imbalance in peripheral blood, spleen and peritoneal lavage from pristane-induced systemic lupus erythematosus (SLE) mice.Adv Rheumatol. 2019 Jul 24;59(1):30. doi: 10.1186/s42358-019-0072-x. Adv Rheumatol. 2019. PMID: 31340848
-
CD4+ T cells in inflammatory diseases: pathogenic T-helper cells and the CD69-Myl9 system.Int Immunol. 2021 Nov 25;33(12):699-704. doi: 10.1093/intimm/dxab053. Int Immunol. 2021. PMID: 34427648 Review.
-
Pathogenesis of Concanavalin A induced autoimmune hepatitis in mice.Int Immunopharmacol. 2022 Jan;102:108411. doi: 10.1016/j.intimp.2021.108411. Epub 2021 Dec 8. Int Immunopharmacol. 2022. PMID: 34891001 Review.
Cited by
-
Novel Therapies for the Treatment of Drug-Induced Liver Injury: A Systematic Review.Front Pharmacol. 2022 Feb 2;12:785790. doi: 10.3389/fphar.2021.785790. eCollection 2021. Front Pharmacol. 2022. PMID: 35185538 Free PMC article. Review.
-
Summary of Natural Products Ameliorate Concanavalin A-Induced Liver Injury: Structures, Sources, Pharmacological Effects, and Mechanisms of Action.Plants (Basel). 2021 Jan 25;10(2):228. doi: 10.3390/plants10020228. Plants (Basel). 2021. PMID: 33503905 Free PMC article. Review.
-
Magnesium isoglycyrrhizinate prevented the liver injury of acetaminophen by promoting mitochondrial biogenesis.Toxicol Res (Camb). 2025 Mar 26;14(2):tfaf024. doi: 10.1093/toxres/tfaf024. eCollection 2025 Apr. Toxicol Res (Camb). 2025. PMID: 40151340
-
NLRP3 inflammasome activation in response to metals.Front Immunol. 2023 Feb 10;14:1055788. doi: 10.3389/fimmu.2023.1055788. eCollection 2023. Front Immunol. 2023. PMID: 36845085 Free PMC article. Review.
-
Jiegeng Decoction Potentiates the Anticancer Efficacy of Paclitaxel in vivo and in vitro.Front Pharmacol. 2022 Feb 25;13:827520. doi: 10.3389/fphar.2022.827520. eCollection 2022. Front Pharmacol. 2022. PMID: 35281908 Free PMC article.
References
-
- Liberal R, Grant CR, Longhi MS, Mieli-Vergani G, Vergani D. Diagnostic criteria of autoimmune hepatitis. Autoimmun Rev. 2014;13(4–5):435–440. - PubMed
-
- Chen J, Eslick GD, Weltman M. Systematic review with meta-analysis: clinical manifestations and management of autoimmune hepatitis in the elderly. Aliment Pharmacol Ther. 2014;39(2):117–124. - PubMed
-
- Floreani A, Liberal R, Vergani D, Mieli-Vergani G. Autoimmune hepatitis: contrasts and comparisons in children and adults – a comprehensive review. J Autoimmun. 2013;46:7–16. - PubMed
-
- Heneghan MA, Yeoman AD, Verma S, Smith AD, Longhi MS. Autoimmune hepatitis. Lancet. 2013;382(9902):1433–1444. - PubMed
-
- Miyara M, Ito Y, Sakaguchi S. TREG-cell therapies for autoimmune rheumatic diseases. Nat Rev Rheumatol. 2014;10(9):543–551. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

