A novel type of self-assembled nanoparticles as targeted gene carriers: an application for plasmid DNA and antimicroRNA oligonucleotide delivery
- PMID: 26869785
- PMCID: PMC4734819
- DOI: 10.2147/IJN.S84927
A novel type of self-assembled nanoparticles as targeted gene carriers: an application for plasmid DNA and antimicroRNA oligonucleotide delivery
Abstract
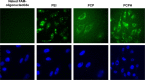
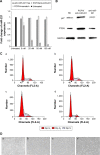
In this study, a new type of amphiphilic cetylated polyethyleneimine (PEI) was synthesized, and then polylactic-co-glycolic acid (PLGA)/cetylated PEI/hyaluronic acid nanoparticles (PCPH NPs) were developed by self-assembly as a novel type of gene-delivering vehicle. The PCPH NPs showed good DNA-condensation ability by forming polyplexes with small particle size and positive zeta potential. The transfection efficiency and cytotoxicity of PCPH NPs were evaluated as plasmid DNA vectors to transfect HepG2 in vitro. PCPH NPs exhibited much lower cytotoxicity and higher gene-transfection efficiency than PEI (25,000) and commercial transfection reagents. Furthermore, PCPH NPs were used as an anti-miR-221 vector for transfecting HepG2 cells, and anti-miR-221 was effectively transfected into cells and produced a greater inhibitory effect on cancer-cell growth by PCPH NPs. These results demonstrate that PCPH NPs can be a promising nonviral vector for gene-delivery systems.
Keywords: PLGA; anti-miR-221; gene delivery; hyaluronic acid; polyethyleneimine.
Figures








Similar articles
-
Complexation of Apoptotic Genes with Polyethyleneimine (PEI)-Coated Poly-(DL)-Lactic-Co-Glycolic Acid Nanoparticles for Cancer Cell Apoptosis.J Biomed Nanotechnol. 2015 Feb;11(2):211-25. doi: 10.1166/jbn.2015.1880. J Biomed Nanotechnol. 2015. PMID: 26349297
-
[Preparation and in vitro evaluation of pDNA-CaPi-PLGA nanoparticles with a core-shell structure].Yao Xue Xue Bao. 2013 Feb;48(2):298-304. Yao Xue Xue Bao. 2013. PMID: 23672030 Chinese.
-
Amphiphilic core-shell nanoparticles containing dense polyethyleneimine shells for efficient delivery of microRNA to Kupffer cells.Int J Nanomedicine. 2016 Jun 15;11:2785-97. doi: 10.2147/IJN.S101251. eCollection 2016. Int J Nanomedicine. 2016. PMID: 27366061 Free PMC article.
-
Progress in microRNA delivery.J Control Release. 2013 Dec 28;172(3):962-74. doi: 10.1016/j.jconrel.2013.09.015. Epub 2013 Sep 25. J Control Release. 2013. PMID: 24075926 Free PMC article. Review.
-
Polymers in small-interfering RNA delivery.Nucleic Acid Ther. 2011 Jun;21(3):133-47. doi: 10.1089/nat.2011.0293. Nucleic Acid Ther. 2011. PMID: 21749290 Free PMC article. Review.
Cited by
-
Comparing the transfection efficiency of cationic monomer ratios in vinylimidazole and aminoethyl methacrylate copolymers.Int J Pharm X. 2025 Mar 4;9:100327. doi: 10.1016/j.ijpx.2025.100327. eCollection 2025 Jun. Int J Pharm X. 2025. PMID: 40124566 Free PMC article.
-
Delivery of therapeutic miRNA using polymer-based formulation.Drug Deliv Transl Res. 2019 Dec;9(6):1043-1056. doi: 10.1007/s13346-019-00645-y. Drug Deliv Transl Res. 2019. PMID: 31049843 Review.
-
Cationic microRNA-delivering nanocarriers for efficient treatment of colon carcinoma in xenograft model.Gene Ther. 2016 Dec;23(12):829-838. doi: 10.1038/gt.2016.60. Epub 2016 Aug 2. Gene Ther. 2016. PMID: 27482839
-
Co-delivery of paclitaxel and TOS-cisplatin via TAT-targeted solid lipid nanoparticles with synergistic antitumor activity against cervical cancer.Int J Nanomedicine. 2017 Jan 31;12:955-968. doi: 10.2147/IJN.S115136. eCollection 2017. Int J Nanomedicine. 2017. PMID: 28203075 Free PMC article.
-
In-vitro Transcribed mRNA Delivery Using PLGA/PEI Nanoparticles into Human Monocyte-derived Dendritic Cells.Iran J Pharm Res. 2019 Fall;18(4):1659-1675. doi: 10.22037/ijpr.2019.1100872. Iran J Pharm Res. 2019. PMID: 32184837 Free PMC article.
References
-
- Verma IM, Weitzman MD. Gene therapy: twenty-first century medicine. Annu Rev Biochem. 2005;74:711–738. - PubMed
-
- Mountain A. Gene therapy: the first decade. Trends Biotechnol. 2000;18(3):119–128. - PubMed
-
- Somia N, Verma IM. Gene therapy: trials and tribulations. Nat Rev Genet. 2000;1(2):91–99. - PubMed
-
- Kim VN, Han J, Siomi MC. Biogenesis of small RNAs in animals. Nat Rev Mol Cell Biol. 2009;10(2):126–139. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

