Miconazole-loaded solid lipid nanoparticles: formulation and evaluation of a novel formula with high bioavailability and antifungal activity
- PMID: 26869787
- PMCID: PMC4734792
- DOI: 10.2147/IJN.S100625
Miconazole-loaded solid lipid nanoparticles: formulation and evaluation of a novel formula with high bioavailability and antifungal activity
Abstract
Background and objective: Miconazole is a broad-spectrum antifungal drug that has poor aqueous solubility (<1 µg/mL); as a result, a reduction in its therapeutic efficacy has been reported. The aim of this study was to formulate and evaluate miconazole-loaded solid lipid nanoparticles (MN-SLNs) for oral administration to find an innovative way to alleviate the disadvantages associated with commercially available capsules.
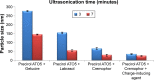
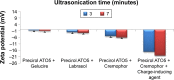
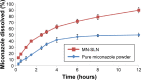
Methods: MN-SLNs were prepared by hot homogenization/ultrasonication. The solubility of miconazole in different solid lipids was measured. The effect of process variables, such as surfactant types, homogenization and ultrasonication times, and the charge-inducing agent on the particle size, zeta potential, and encapsulation efficiency were determined. Furthermore, in vitro drug release, antifungal activity against Candida albicans, and in vivo pharmacokinetics were studied in rabbits.
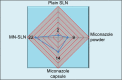
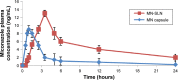
Results: The MN-SLN, consisting of 1.5% miconazole, 2% Precirol ATO5, 2.5% Cremophor RH40, 0.5% Lecinol, and 0.1% Dicetylphosphate, had an average diameter of 23 nm with a 90.2% entrapment efficiency. Furthermore, the formulation of MN-SLNs enhanced the antifungal activity compared with miconazole capsules. An in vivo pharmacokinetic study revealed that the bioavailability was enhanced by >2.5-fold.
Conclusion: MN-SLN was more efficient in the treatment of candidiasis with enhanced oral bioavailability and could be a promising carrier for the oral delivery of miconazole.
Keywords: Cremophor RH40; Precirol ATO5; antifungal activity; encapsulation; miconazole; solid lipid nanoparticles.
Figures
Similar articles
-
Sildenafil citrate as oral solid lipid nanoparticles: a novel formula with higher bioavailability and sustained action for treatment of erectile dysfunction.Expert Opin Drug Deliv. 2014 Jul;11(7):1015-22. doi: 10.1517/17425247.2014.912212. Epub 2014 Apr 19. Expert Opin Drug Deliv. 2014. PMID: 24746063
-
Solid lipid nanoparticles loaded with iron to overcome barriers for treatment of iron deficiency anemia.Drug Des Devel Ther. 2015 Jan 8;9:313-20. doi: 10.2147/DDDT.S77702. eCollection 2015. Drug Des Devel Ther. 2015. PMID: 25609917 Free PMC article.
-
Influence of formulation variables on miconazole nitrate-loaded lipid based nanocarrier for topical delivery.Colloids Surf B Biointerfaces. 2020 Sep;193:111046. doi: 10.1016/j.colsurfb.2020.111046. Epub 2020 May 1. Colloids Surf B Biointerfaces. 2020. PMID: 32416518
-
Solid lipid nanoparticles: an oral bioavailability enhancer vehicle.Expert Opin Drug Deliv. 2011 Nov;8(11):1407-24. doi: 10.1517/17425247.2011.604311. Epub 2011 Aug 11. Expert Opin Drug Deliv. 2011. PMID: 21831007 Review.
-
Nano-Sized Technologies for Miconazole Skin Delivery.Curr Pharm Biotechnol. 2016;17(6):524-31. doi: 10.2174/1389201017666160301102459. Curr Pharm Biotechnol. 2016. PMID: 26927217 Review.
Cited by
-
Development and Evaluation of Solid Lipid Nanoparticles for the Clearance of Aβ in Alzheimer's Disease.Pharmaceutics. 2023 Jan 9;15(1):221. doi: 10.3390/pharmaceutics15010221. Pharmaceutics. 2023. PMID: 36678849 Free PMC article.
-
In Vivo Evaluation of Miconazole-Nitrate-Loaded Transethosomal Gel Using a Rat Model Infected with Candida albicans.Pharmaceuticals (Basel). 2024 Apr 24;17(5):546. doi: 10.3390/ph17050546. Pharmaceuticals (Basel). 2024. PMID: 38794118 Free PMC article.
-
Synthesis of Retinol-Loaded Lipid Nanocarrier via Vacuum Emulsification to Improve Topical Skin Delivery.Polymers (Basel). 2021 Mar 8;13(5):826. doi: 10.3390/polym13050826. Polymers (Basel). 2021. PMID: 33800335 Free PMC article.
-
Preparation and evaluation of tacrolimus-loaded thermosensitive solid lipid nanoparticles for improved dermal distribution.Int J Nanomedicine. 2019 Jul 18;14:5381-5396. doi: 10.2147/IJN.S215153. eCollection 2019. Int J Nanomedicine. 2019. PMID: 31409994 Free PMC article.
-
Development, optimization, and evaluation of a nanostructured lipid carrier of sesame oil loaded with miconazole for the treatment of oral candidiasis.Drug Deliv. 2022 Dec;29(1):254-262. doi: 10.1080/10717544.2021.2023703. Drug Deliv. 2022. PMID: 35014929 Free PMC article.
References
-
- Bensadoun R-J, Daoud J, El Gueddari B, et al. Comparison of the efficacy and safety of miconazole 50-mg mucoadhesive buccal tablets with miconazole 500-mg gel in the treatment of oropharyngeal candidiasis. Cancer. 2008;112(1):204–211. - PubMed
-
- Mendes AI, Silva AC, Catita JA, et al. Miconazole-loaded nanostructured lipid carriers (NLC) for local delivery to the oral mucosa: improving antifungal activity. Colloids Surf B Biointerfaces. 2013;111:755–763. - PubMed
-
- Pedersen M, Edelsten M, Nielsen VF, et al. Formation and antimycotic effect of cyclodextrin inclusion complexes of econazole and miconazole. Int J Pharm. 1993;90(3):247–254.
-
- Jain S, Jain S, Khare P, et al. Design and development of solid lipid nanoparticles for topical delivery of an anti-fungal agent. Drug Deliv. 2010;17(6):443–451. - PubMed
-
- Fothergill AW. Miconazole: a historical perspective. Expert Rev Anti Infect Ther. 2006;4(2):171–175. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

