The COLOFOL trial: study design and comparison of the study population with the source cancer population
- PMID: 26869813
- PMCID: PMC4734721
- DOI: 10.2147/CLEP.S92661
The COLOFOL trial: study design and comparison of the study population with the source cancer population
Abstract
Introduction: The COLOFOL trial, a prospective randomized multicenter trial comparing two follow-up regimes after curative surgical treatment for colorectal cancer, focuses on detection of asymptomatic recurrences. This paper aims to describe the design and recruitment procedure in the COLOFOL trial, comparing demographic characteristics between randomized patients and eligible patients not included in the study.
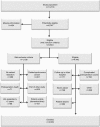
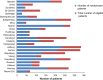
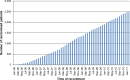
Materials and methods: COLOFOL was designed as a pragmatic trial with wide inclusion criteria and few exclusion criteria, in order to obtain a sample reflecting the general patient population. To be eligible, patients had to be 75 years or younger and curatively resected for stage II or III colorectal cancer. Exclusion criteria were hereditary colorectal cancer, no signed consent, other malignancy, and life expectancy less than 2 years due to concomitant disease. In four of the 24 participating centers, we scrutinized hospital inpatient data to identify all colorectal cancer patients who underwent surgery, in order to ascertain all eligible patients who were not included in the study and to compare them with enrolled patients.
Results: Of a total of 4,445 eligible patients, 2,509 patients were randomized (56.4% inclusion rate). A total of 1,221 eligible patients were identified in the scrutinized hospitals, of which 684 (56%) were randomized. No difference in age or sex distribution was observed between randomized and nonrandomized eligible patients. However, a difference was noted in tumor location and stage distribution, with 5.6% more patients in the randomized group having colon cancer and 6.7% more patients having stage II disease.
Conclusion: Patients in the two study arms were not only demographically similar, but also similar to nonincluded eligible patients, apart from stage and localization. The analyses will be stratified by these variables. Taken together, we conclude that our trial results will be robust and possible to extrapolate to the target population.
Keywords: colorectal cancer; follow-up; source population; trial design.
Figures
References
-
- Baca B, Beart RW, Jr, Etzioni DA. Surveillance after colorectal cancer resection: a systematic review. Dis Colon Rectum. 2011;54(8):1036–1048. - PubMed
-
- Komborozos VA, Skrekas GJ, Pissiotis CA. The contribution of follow-up programs in the reduction of mortality of rectal cancer recurrences. Dig Surg. 2001;18(5):403–408. - PubMed
-
- Rodriguez-Moranta F, Salo J, Arcusa A, et al. Postoperative surveillance in patients with colorectal cancer who have undergone curative resection: a prospective, multicenter, randomized, controlled trial. J Clin Oncol. 2006;24(3):386–393. - PubMed
-
- Secco GB, Fardelli R, Gianquinto D, et al. Efficacy and cost of risk-adapted follow-up in patients after colorectal cancer surgery: a prospective, randomized and controlled trial. Eur J Surg Oncol. 2002;28(4):418–423. - PubMed
-
- Makela JT, Laitinen SO, Kairaluoma MI. Five-year follow-up after radical surgery for colorectal cancer. Results of a prospective randomized trial. Arch Surg. 1995;130(10):1062–1067. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources